Such a useful medicine, paracetamol - I suppose it’s been around as long as aspirin?
No, it didn’t come into use until the second half of the 20th century, nearly a hundred years after aspirin (see MOTM for February 1996) – and it wasn’t the first drug of the type, either.
Tell me more
The chemical industry grew from virtually nothing in the second half of the 19th century. It stemmed from 18-year old William Henry Perkin making a serendipitous discovery in 1856; he was trying to make the antimalarial drug, quinine (see MOTM for July 2005), but instead succeeded in making mauveine, the first synthetic dye (see MOTM for Jan 1996).
Over the next 20-30 years, the German chemical industry grew enormously. They used coal as the raw material; on distilling coal away from air, they obtained coal gas, used for domestic and industrial heating and lighting (electric light did not exist at that time). During the 19th century, all towns and cities acquired their own “gas works”; they prominently featured ‘gas holders’ (gasometers) which stored the coal gas en route to being piped to consumers. These gas works have disappeared over the past fifty years because ‘natural gas’, largely methane, piped from offshore rigs, has become the norm for domestic heating and cooking.
| 
Paracetamol is one of the most popular choices
of painkillers, especially for headaches.
[Photo: Designed by Freepik]
|
As well as coal gas, ‘gas works’ also made coke (used for heating and by the iron and steel industry; ammonia; and coal tar - a viscous black liquid that contains a range of different molecules used to make dyestuffs.
 |
 |
Coal tar - a black treacle-like liquid that is used to paint walls and roofs.
It is odd to think that so many useful chemicals, including dyes and paracetamol,
originate from this gooey fluid. |
Disused gasworks in Seattle.
[Photo: Joe Mabel (CC BY-SA) via Wikimedia Commons |
One of the products that was first isolated from coal tar in 1834 was phenylamine (aniline). In 1854, Antoine Béchamp discovered that it could be made cheaply from nitrobenzene by reduction with iron and hydrochloric acid (the Béchamp reaction).
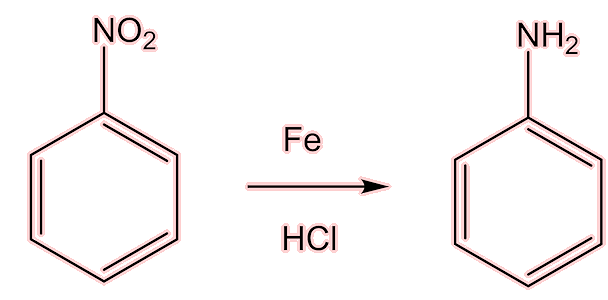
This discovery was the key to fabricating cheap, synthetic pharmaceuticals, leading very quickly to the first of the 'coal tar' painkillers.
Which was?
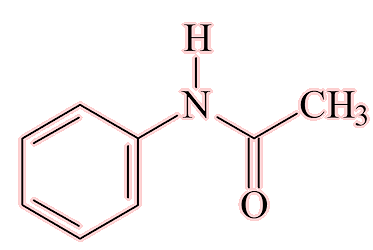
Acetanilide (N-phenylethanamide) was the first, as a result of a(nother) piece of serendipity. Arnold Chan and Paul Hepp, two researchers at the University of Strasbourg, were investigating intestinal worms in patients. They ordered the standard chemical used to treat them, naphthalene, but the wholesalers sent them acetanilide by mistake. This was a by-product from the coal-tar industry. They found that it was useless as a treatment for worms, but did lower the temperature of some feverish patients. Hepp’s brother was a chemist with Kalle and Company, and saw the potential of this cheap and easy-to-make substance. Acetanilide was a known chemical, so it could not be patented, so in 1886 they marketed acetanilide as ‘Antifebrin’, a medicine with analgesic (pain-killing) and antipyretic (temperature-lowering) properties.
And it is easy to make?
Yes, simply by the acylation of aniline, either with ethanoic anhydride (below) or ethanoyl chloride.
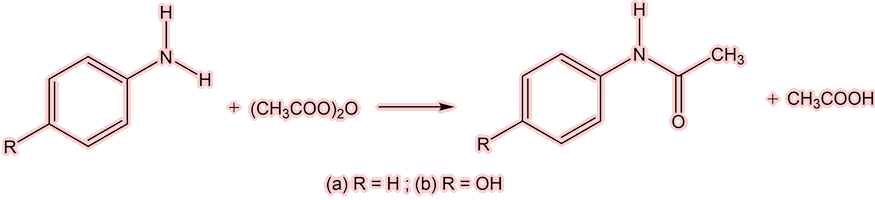
The acylation of aniline to give either (a) R = H, with product acetanilide, or (b) R = OH, and the product paracetamol.
Acylation was one of the important new reaction types discovered in the 19th century. It was used about a decade after acetanilide’s discovery to make acetylsalicylic acid (aspirin) from salicylic acid, and, less felicitously, to make diacetylmorphine (heroin) from morphine.
And acetanilide was a success?
Not for long. It was discovered that it oxidised haemoglobin and reduced the oxygen-carrying capacity of the blood (methaemoglobia), as well as causing liver and kidney damage. So, back to square one...
And then?
Well, the next of the painkillers came on the scene almost at once. The Bayer company (Farbenfabriken Bayer) had a lot of a dye-waste product, para-nitrophenol (which we now call 4-nitrophenol). They knew that, like nitrobenzene, it could be reduced to 4-aminophenol, then acetylated to make acetaminophen(ol) [see later!] and then converted into 4-ethoxyacetanilide by the Williamson synthesis for ethers.
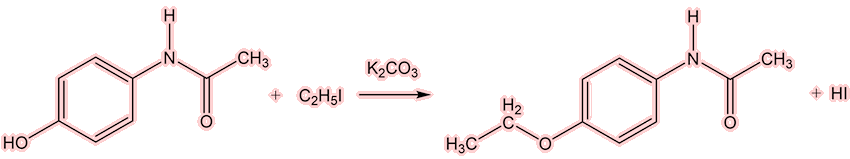
Acetaminophen is turned to the aryloxide ion (shown below as RO-) under basic conditions; the aryloxide carries out a nucleophilic attack upon the iodoethane.
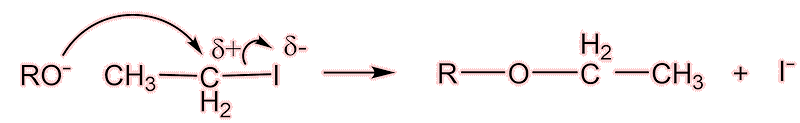
4-ethoxyacetanilide was also found to have analgesic and antipyretic properties and put onto the market in 1887-8 as Phenacetin.
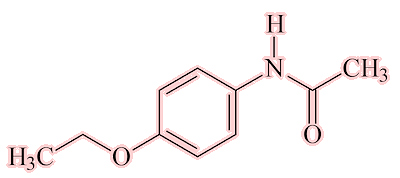
Phenacetin
And it was a success?
Indeed. Until the 1960s it was used world-wide. Like paracetamol today, it was often used in combination with codeine (and other medicines, including caffeine). However, it was found to cause kidney damage and was also a carcinogen, and this is what caused its eventual withdrawal from use. Like acetanilide, it also caused methaemoglobia.
Could it kill people?
Howard Hughes (1905-1976) is believed to have been one victim of phenacetin. He was a quite remarkable man, with an incredibly diverse range of interests. You may recall the Hollywood movie The Aviator, starring Leonardo di Caprio, which was made about his life. Hughes was an entrepreneur in the chemical industry, but was also involved in making films and designing aircraft – and test-flying them. The ‘Spruce Goose’ flying boat, one of the largest airplanes ever built, was one of his creations.

The ‘Spruce Goose’ flying boat
Photo: Public domain via Wikimedia Commons
On July 7th 1946, Hughes was testing an experimental reconnaissance plane, the XF-11, which crashed soon after take-off from the airport of Santa Monica, in California. Somehow he survived, but with life-threatening injuries (3rd-degree burns and multiple fractures). He needed four skin drafts and three chest drainings. He was on morphine (MOTM November 2004) throughout his five-week hospitalisation and was left with intractable (chronic) pain for the rest of his life. He took a daily dose of 1200-2700 mg codeine (MOTM December 2015); sometimes he took it in combination with phenacetin, and it is believed that long-term use – 30 years - of phenacetin caused the kidney failure which killed him. |

Howard Hughes
Photo: Acme Newspictures / Public domain
|
So what happened next?
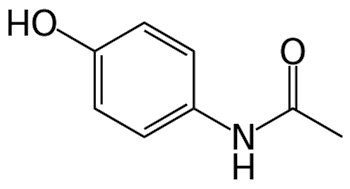
Paracetamol happened.
It had first been made in 1877. In the late 19th century, it was believed that phenacetin was safer than paracetamol, which is why paracetamol was not taken further. In the 1940s it was found that phenacetin and acetanilide owe their beneficial effects to acetaminophen, formed as a metabolite in the body, in other words, they acted as prodrugs. And acetaminophen had none of their side-effects.
|
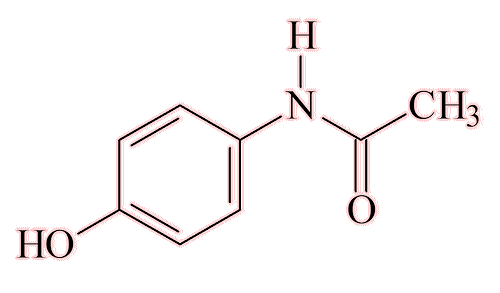
Paracetamol (acetaminophen)
|
During the 1950s, an American firm, McNeil Laboratories, started to market acetaminophen medication as a product called Tylenol, and to this day this is how it is known in the USA. Its use spread everywhere. It was marketed in the UK from 1956 as Panadol, but the generic form, as patent protection has long expired, is known as Paracetamol. It is used on its own, as well as in combination with other substances, particularly codeine, and today it is a cheap, over-the-counter medication found in virtually every home.
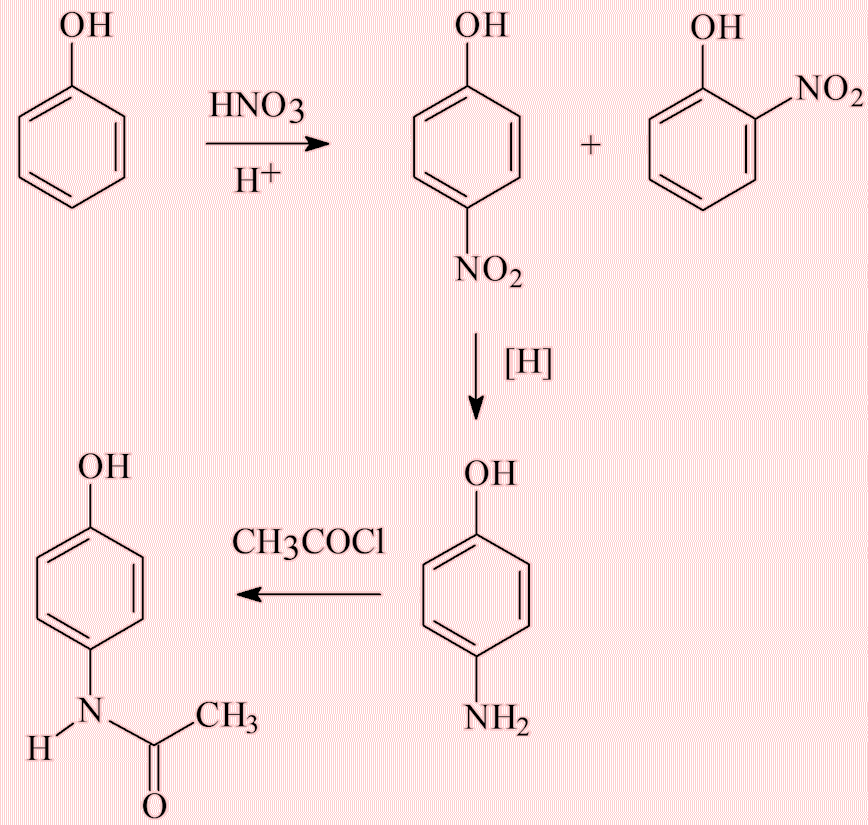
How do you make it?
In a very similar way to acetanilide (see under acetanilide, with R = OH). Going back to phenol, its nitration gives two products, the ortho- (2-) and para- (4-) nitrophenols. They can be separated by steam distillation, and the 4-nitrophenol reduced to 4-aminophenol, which can then be acetylated. All the chemicals involved are inexpensive, which is why paracetamol is so cheap.
How does paracetamol work?
One theory is that some of it is metabolised in the body to 4-aminophenol. This can link with the fatty acid arachidonic acid forming N-arachidonoylphenolamine (AM404). This is a naturally occurring cannabinoid (a group of molecules which activates specific receptors in the brain and spinal cord, and whose most famous member is tetrahydrocannabinol (THC), the primary psychoactive compound found in cannabis (see MOTM for April 2019). AM404 is thought to bind to the cannabinoid CB1 receptors and blocks them, preventing other molecules binding there and triggering pain signals. So, it effectively 'switches off' the pain signals.
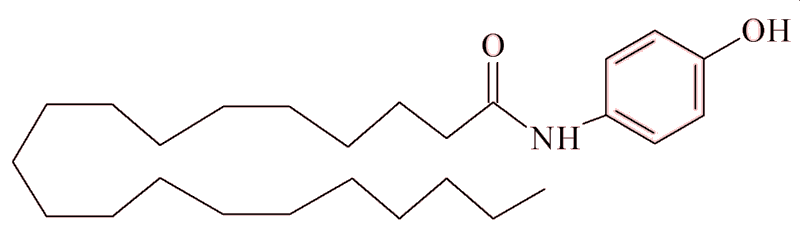
AM404 (N-arachidonoylaminophenol)
Most of a normal dose of paracetamol is metabolised into harmless metabolites in the body, but some 5% is converted into N-acetyl-p-benzoquinone imine (NAPQI), which is believed to bind to receptors stopping the COX-2 enzyme forming inflammatory compounds. So paracetamol both helps to relieve pain and reduce inflammation, as well as lower fever.
How does paracetamol shape up against aspirin?
Unlike aspirin, acetaminophen is not an anti-inflammatory medication. Also, unlike aspirin, it does not act as a stomach irritant (as it is not acidic), but too much of it causes liver damage.
It is not paracetamol that causes the damage, but the 5% of it that the body changes to the the quinoneimine (NAPQI). The body contains a limited amount of glutathione (GSH) that mops up small amounts of NAPQI. But an overdose of paracetamol uses up the body’s stock of GSH, and the excess NAPQI attacks the liver, often with fatal liver failure, along with kidney failure. Its low price and easy availability sadly make paracetamol overdose one of the favourite methods for suicide attempts in developed countries. Indeed, paracetamol overdoses (both deliberate and accidental) are responsible for some 30,000 to 40,000 visits to Casualty departments a year in the UK, with around 200 fatalities. Many pharmacies and shops now limit the sales of paracetamol to only one box per customer per visit, to try to reduce this risk.
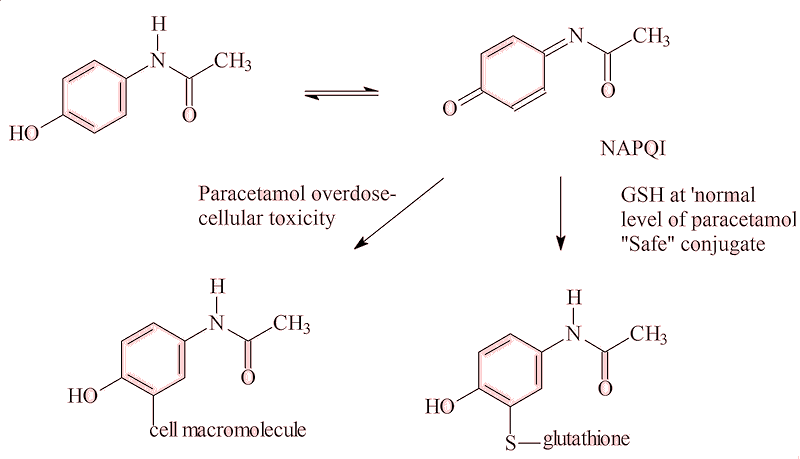
Is there an antidote?
Both L-methionine and N-acetyl-L-cysteine can enable the human body to overcome such an overdose, probably by promoting the synthesis of GSH. So one safeguard would be to add some methionine to every paracetamol tablet, but few manufacturers do this due to the added cost.
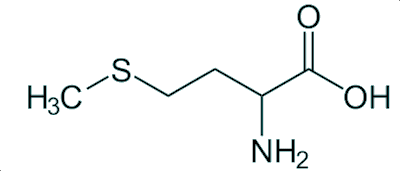
L-methionine
 A problem with paracetamol overdoses is that the antidotes ideally have to be administered within 2 hours, certainly within 8 to 10 hours. Part of the problem with paracetamol overdoses is that there are few symptoms in the early stages, and someone having a change of heart after deliberately taking an overdose may think that the lack of any apparent symptoms means there is no reason to seek treatment – fatally – because liver failure symptoms can take 16-36 hours to appear.
A problem with paracetamol overdoses is that the antidotes ideally have to be administered within 2 hours, certainly within 8 to 10 hours. Part of the problem with paracetamol overdoses is that there are few symptoms in the early stages, and someone having a change of heart after deliberately taking an overdose may think that the lack of any apparent symptoms means there is no reason to seek treatment – fatally – because liver failure symptoms can take 16-36 hours to appear.
So paracetamol can be a killer?
Not if you take the dose given on the packet.
What about the “Tylenol murders”
These weren’t actually caused by paracetamol. Early on September 29th 1982, 12-year-old Mary Kellerman complained to her parents of a runny nose and sore throat. They gave her a Tylenol capsule. Unknown to her parents, someone had introduced potassium cyanide into that capsule, and that killed her. Within days, six other people, who like Mary lived in the area of Chicago, died in the same way. Tylenol was still manufactured by McNeil Laboratories, by then a subsidiary of Johnson and Johnson, an American-based multinational corporation dealing particularly in medical and pharmaceutical products. The manufacturers acted promptly, withdrawing over 31 million bottles of Tylenol from circulation (and offering replacements to consumers). Three more bottles of cyanide-laced capsules were found on shop shelves. It was established that the contamination had taken place in shops, not during manufacture, but the perpetrator was never established. The most important consequence is that the manufacturers subsequently introduced tamper-proof packaging.

A brown tree snake on Guam
Photo: USDA |
I've heard that Paracetamol is being used to kill snakes?
Thanks to the American military’s involuntary efforts, brown tree-snakes (Boiga irregularis) arrived on the Pacific island of Guam in the 1940s. Sadly, they have consumed much of the island’s bird life, so in recent years a cunning plan has been put in place. The plan is to use paracetamol-baited dead mice, dropped by parachute. Around 80 mg of paracetamol is a lethal dose to snakes (it is also lethal to cats, but not to dogs), and dropping the mice so they land in the trees should stop other wildlife from being poisoned. Laboratory tests find that the cause of the snakes’ death is acute methemoglobinemia and respiratory failure; this is believed to be a humane method killing the snakes.
|

Bibliography
- Chapman and Hall Combined Chemical Dictionary code numbers: FFL96-T (paracetamol); JQS51-C (acetanilide); BLS33-F (phenacetin)
- A. Cahn and P. Hepp, Centralblatt für klinische Medizin, 1886, 7, 561–564 (original synthesis of acetanilide (Antifebrin))
- H. N. Morse, Berichte der deutschen chemischen Gesellschaft, 1878, 11, 232-233 (original synthesis of phenacetin)
- D. Lester, L. A. Greenberg and R. P. Carroll, J. Pharmacol. Exp. Ther. 1947, 90, 68–75; B. B. Brodie and J. Axelrod, J. Pharmacol. Exp. Ther., 1948, 94, 22–28; idem, 29–38; F. B. Flinn and B. B. Brodie, J. Pharmacol. Exp. Ther., 1948, 94, 76-77 (metabolism of acetanilide in the body)
- B. B. Brodie and J. Axelrod, J. Pharmacol. Exp. Ther., 1948, 94, 58-67 (metabolism of phenacetin)
- G. Margetts, J. Int. Med. Res., 1976, 4, Supplement (4), 55-70 (phenacetin, paracetamol and the liver)
- M. Beck, S. Monroe, L. R. Prout, M. Hager and R. LaBreque, Newsweek, October 11, 1982, p. 32 (the Tylenol murders)
- A.L. Jones, P. C. Hayes, A. T. Proudfoot, J. A. Vale and L. F. Prescott, Brit. Med. J. (Clin. Res. Ed.), 1997, 315, 301–303 (should methionine be added to every paracetamol tablet?)
- J. J. Johnston, P. J. Savarie, T. M. Primus, J. D. Eisemann, J. C. Hurley and D. J. Kohler, Environ. Sci. Technol., 2002, 36, 3827– 3833. (Paracetamol and brown snakes)
- K. D. Rainsford (ed.), Aspirin and Related Drugs, London and New York, Taylor and Francis, 2004, esp. pp. 181-213 (Paracetamol).
- D. Bartlett, J. Emerg. Nurs., 2004, 30, 281–283. (Treatment for overdoses)
- E. D. Högestätt, et al. J. Biol. Chem., 2005, 280, 31405–31412. (AM404)
- A. Ottani, S. Leone, M. Sandrini, A. Ferrari and A. Bertolini, Euro. J. Pharmacol., 2006, 531, 80–281. (Paracetamol and cannabinoid CB1 receptors)
- A. Bertolini, A. Ferrari, A. Ottani, S. Guerzoni, R. Tacchi and S. Leone, CNS Drug Reviews, 2006, 12, 250–275. (review)
- G. B. Steventon and A. Hutt, in R. H. Waring, G. B. Steventon and S. C. Mitchell (eds), Molecules of Death, 2nd edition, Imperial College Press, London, 2007, pp. 253–263.
- F. Tennant, Howard Hughes and Pseudoaddiction, Practical Pain Management, July/August 2007, 12-29 (Howard Hughes’ injuries and medication)
- B. Anderson, J. Pediatric Anesthesia, 2008, 18, 915–921 (How paracetamol acts)
- D. A. Andersson et al., Nat. Commun., 2011, 2, 551 (Paracetamol metabolites activate receptors in spinal cord)
- A. W. Jones, Drug Test. Analysis, 2011, 3, 337–344 (“Early drug discovery and the rise of pharmaceutical chemistry”)
- G. G. Graham, M. J. Davies, R. O. Day, A. Mohamudally and K. F. Scott, Inflammopharmacol., 2013, 21, 201–232 (paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings)
- M. Jóźwiak-Bębenista and J. Z. Nowak, Acta Poloniae Pharmaceutica - Drug Research, 2014, 71, 11-23 (paracetamol: mechanism of action, applications and safety concerns)
- T. Mathies and R. E. Mauldin, Sci. Rep., 2020, 10, 845 (‘Lethal methemoglobinemia in the invasive brown treesnake after acetaminophen ingestion")
- http://cen.acs.org/articles/91/i9/Chemical-Characters-Dead-Mouse-Trap.html (paracetamol on Guam)
- Time magazine: summary of the Tylenol poisonings


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12181980]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12181980]
![]()
![]()
![]()
![]()







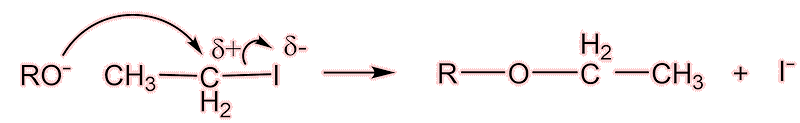









 A problem with paracetamol overdoses is that the antidotes ideally have to be administered within 2 hours, certainly within 8 to 10 hours. Part of the problem with paracetamol overdoses is that there are few symptoms in the early stages, and someone having a change of heart after deliberately taking an overdose may think that the lack of any apparent symptoms means there is no reason to seek treatment – fatally – because liver failure symptoms can take 16-36 hours to appear.
A problem with paracetamol overdoses is that the antidotes ideally have to be administered within 2 hours, certainly within 8 to 10 hours. Part of the problem with paracetamol overdoses is that there are few symptoms in the early stages, and someone having a change of heart after deliberately taking an overdose may think that the lack of any apparent symptoms means there is no reason to seek treatment – fatally – because liver failure symptoms can take 16-36 hours to appear.