|

Ketene
The molecule linked with
vaping-associated lung injury (EVALI)

Simon Cotton
University of Birmingham

Molecule of the Month June 2025
Also available: HTML version.

|

[Image: TBEC Review, CC BY 2.0 via Wikimedia Commons ] |
Don’t you mean ketone?
No, the molecule shown is much more than a ketone; it also contains the C=C bond found in alkenes.
But it is a strange name...
Its systematic name is ethenone, as it is an unsaturated ketone which contains two carbon atoms. It is like an ethene molecule (MOTM December 2006) in which two hydrogen atoms have been replaced by an oxygen, creating a carbonyl group.
What’s ketene like?
It is a very reactive (and toxic) colourless gas, with a boiling point of -56°C. Above -80°C, it readily oligomerises, either to the dimer (diketene) or polymers. The dimer breaks down to the monomer on heating to 550°C.
|
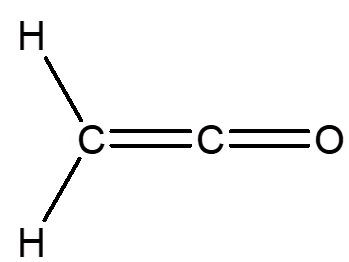
Ketene |
|
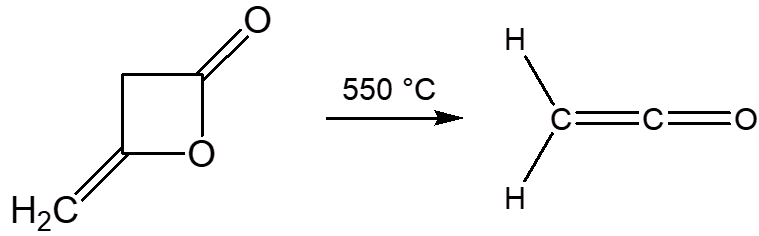
How is ketene made?
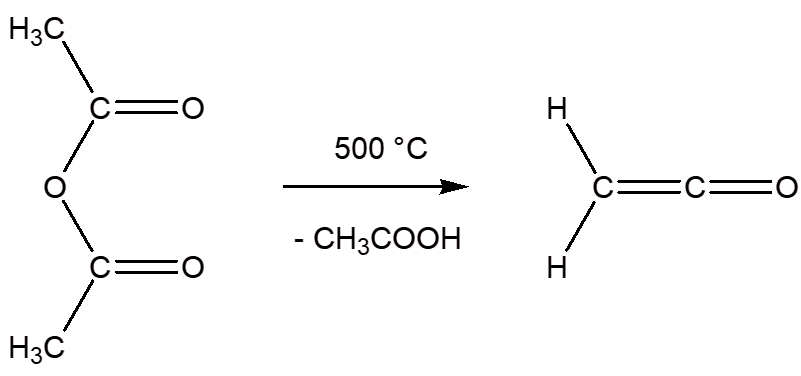
It can be got by various routes, such as heating ethanoic anhydride (acetic anhydride) to 500°C, whereupon it eliminates ethanoic acid.
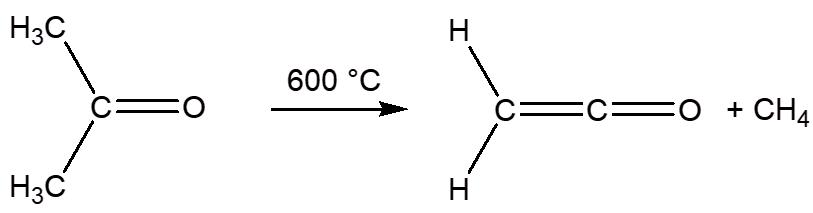
Likewise, it is formed by thermolysis of propanone (acetone) at 600-700°C.
How reactive is ketene?
Very. It readily undergoes addition reactions across the C=C bond, as you would expect from its structure:
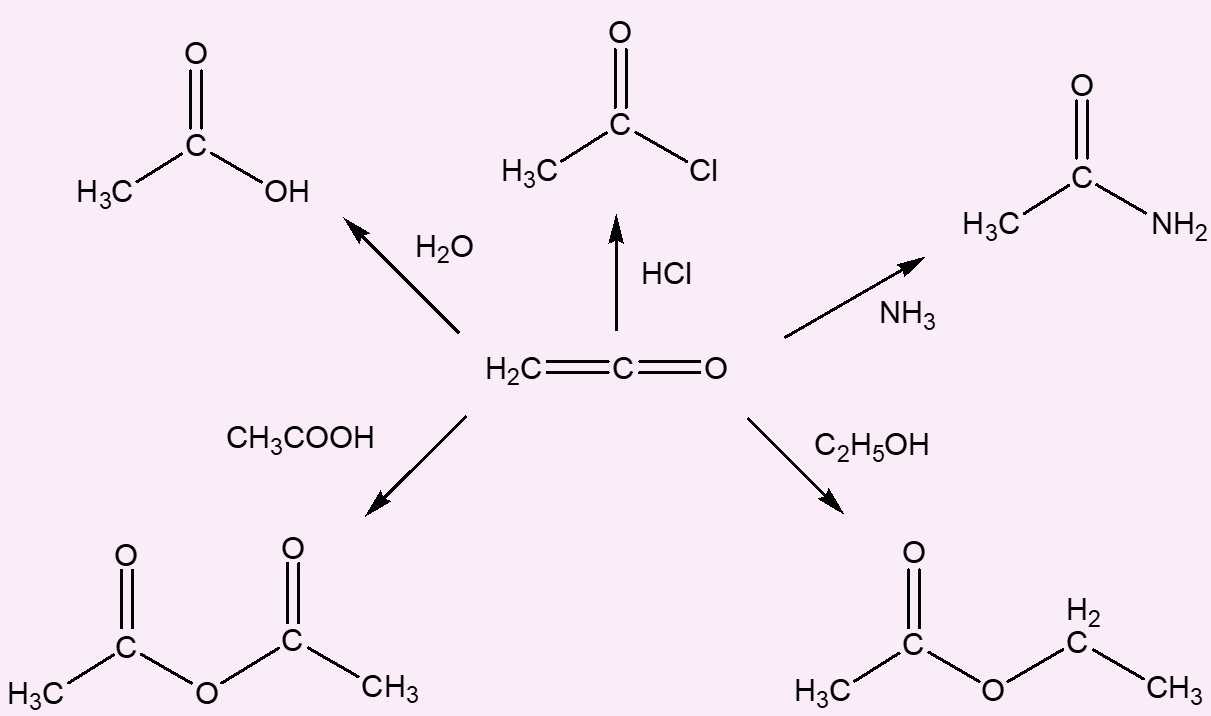
Thus it undergoes additions with water forming ethanoic acid (acetic acid); with hydrogen halides forming ethanoyl halides; with ammonia forming ethanamide (acetamide); with ethanoic acid forming ethanoic anhydride (acetic anhydride); and with alcohols forming esters.
How toxic is ketene?
Ketene is particularly toxic to the pulmonary system, reportedly more toxic than phosgene (COCl2), a chemical warfare agent used in World War I, associated with some 85,000 deaths.
Why has ketene been in the news?
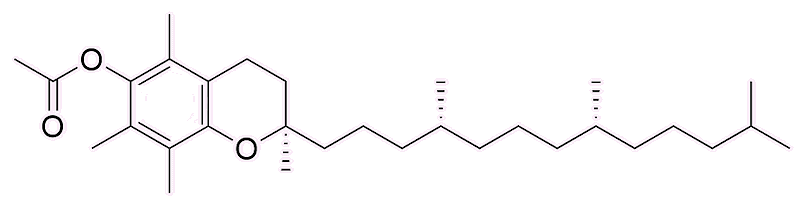
In 2019 there was an outbreak of a serious lung disease in the USA known as 'vaping-associated pulmonary illness' (VAPI), which later became known as 'E-cigarette/vaping-product-use-associated lung injury' (EVALI). As of February 28th 2020, some 2807 hospitalisations were associated with the disease, with 68 fatalities; after that date, because of the rapid rise of COVID-19, it was difficult to distinguish between EVALI and COVID-19. It was soon evident that the disease was related to the use of e-cigarettes and vaping, notably those involving tetrahydrocannabinol (see MOTM April 2019) oil. The acetate of vitamin E (see MOTM for September 2016), a.k.a. α-Tocopheryl acetate), became associated with the complaint, added to the e-liquid as a thickening agent to assist in vaping. THC-O-acetate is a schedule-I drug in the United States, but was widely advertised on websites. Cases of EVALI are very rare outside of the USA.
|

Washington Post article about EVALI |
 |
|
| Vitamin E acetate (a.k.a. α-Tocopheryl acetate) |
|
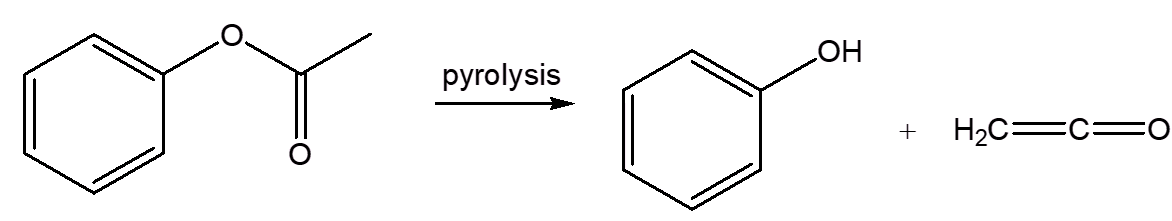
It was already known that pyrolysis of phenyl acetate affords the toxic and highly reactive unsaturated ketone, ketene.
Vitamin E acetate also contains an acetate group attached to a benzene ring. Experiments showed that heating Vitamin E acetate also gave rise to ketene. Moreover, Δ9-THC acetate, an unregulated cannabinoid used in cannabis vapes, which accumulates in the alveoli causing lipoid pneumonia and disrupting the pulmonary surfactant, similarly forms ketene under vaping conditions.
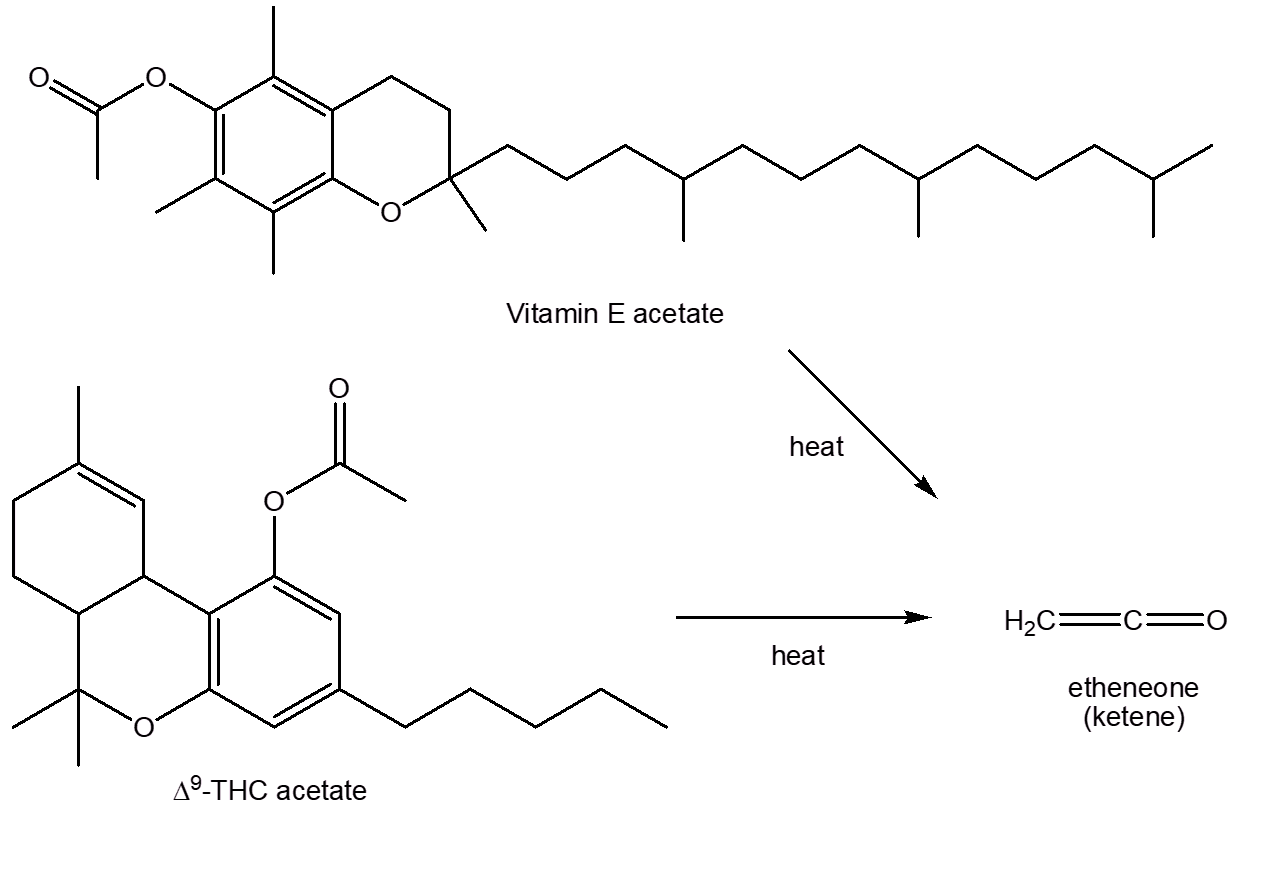
These decomposition reactions may be aided by the metal in the heating element in e-cigarettes – metals such as copper are known to catalyse the formation of ketene in the thermal decomposition of molecules like propanone.
So whilst not proven, it looks very likely that ketene is responsible for EVALI.

Bibliography
- Chapman and Hall Combined Chemical Dictionary code number: CVQ98-V
- N.T.M. Wilsmore, J. Chem. Soc., 1907, 91, 1938-1941 (synthesis)
- K. Blau, Chem. Ind. (London), 1963, 33-34 (synthesis)
- T. T. Tidwell, Ketenes, Hoboken, NJ, Wiley Interscience, 2nd ed. 2006.
Reviews
- T. T. Tidwell, Angew. Chem. Int. Ed. 2005, 44, 5778-5785.
- T. T. Tidwell, Eur. J. Org. Chem., 2006, 563-576.
- A. D. Allen and T. T. Tidwell, ARKIVOC, 2016, (i) 415-490.
Safety
- L. Bretherick, Handbook of Reactive Chemical Hazards, 4th ed., Butterworths, 1990, 0680.
- S. G. Luxon, Hazards in the Chemical Laboratory, 5th ed., Royal Society of Chemistry, 1992, 764.
- R. J. Lewis, Sax's Dangerous Properties of Industrial Materials, 8th ed., Van Nostrand Reinhold, 1992, KEU000
Vitamin E acetate, cannabinoid acetates and EVALI
- A.K. Werner, et al., N. Engl. J. Med., 2020, 382, 1589–1598 (hospitalizations and deaths associated with EVALI)
- S. G. Doukas, L. Kavali, R. S. Menon, B. N. Izotov and A. Bukhari, Toxicol. Rep., 2020, 7, 1381–1386 (review of EVALI)
- B. C. Blount, et al., N. Engl. J. Med., 2020, 382, 697–705 (Vitamin E acetate associated with EVALI)
- D. Wu and D. F. O’Shea, PNAS, 2020, 117, 6349–6355; R. M. Strongin, PNAS, 2020, 117, 7553–7554 (formation of ketene from Vitamin E acetate)
- K. R. Attfield, W. Chen, K. J. Cummings, P. Jacob, D. F. O’Shea, J. Wagner, P. Wang and
J. Fowles, Am. J. of Respir. and Crit. Care Med., 2020, 202, 1187-1188 (potential of ethenone (ketene) to contribute to EVALI)
- K. R. Munger, R. P. Jensen and R. M. Strongin, Chem. Res. Toxicol. 2022, 35, 1202–1205 (vaping cannabinoid acetates leads to ketene formation)
- M. E. Rebuli, et al., Ann. Am. Thorac. Soc., 2023, 20, 1–17 (EVALI and e-cigarette or vaping product use)
- K. R. Munger, K. M. Anreise, R. P. Jensen, D. H. Peyton and R. M. Strongin, JACS Au, 2024, 4, 2403−2410 (ketene formation from cannabinoid acetates)
EVALI and Vitamin E acetate


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27179136]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27179136]
![]()
![]()
![]()
![]()









