
Luciferin
The glowing group of molecules
responsible for bioluminescence

Leila Hewitt, Elise Lacey, Columbus Layton and Emilia White
Hereford Sixth Form College

Molecule of the Month November 2019
Also available: JSMol version.

|

A firefly (above) and the molecule of
luciferin (below) responsible for its light.
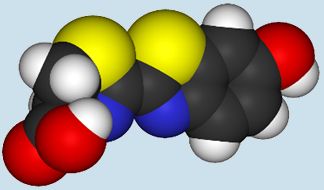
|
Luciferin? Why such a hellish name?
The name of this group of incredible glow-inducing compounds, found in many different bioluminescent organisms, comes from the Latin lucifer meaning ‘light bringer’, so the name of these chemicals has much more to do with their unusual properties than any reference to the devil (phew).
Biolumi-what?
Yes, it’s a rather ostentatious term: bioluminescence, but really it just describes the process by which a living organism emits light, biochemically. Often the stars of nature and science documentaries, bioluminescent organisms are surprisingly common, with more than three quarters of marine animals having such luminous powers. Some examples are fireflies, some snails, zooplankton, and several species of jellyfish and sea anemones. Despite being first described by ancient civilizations, the magnificent mystery of bioluminescence remained unexplained for millennia, until its chemical secret was discovered.
"
Bioluminescent dinoflagellates (Lingulodinium polyedrum) lighting a breaking wave at midnight.
Photo: Mike from: https://www.flickr.com/photos/58761930@N00/206368807/ via CC BY-SA 2.0 licence.
How did ideas about bioluminescence emerge?
There has been lots of superstition surrounding bioluminescence for a long time. For example, the myths of the lore of ancient seafarers mentioned the phenomenon several times. Stories arose of unusual lights or fires gathering over water, fields or mountains. At the time, the only explanation for these mysterious events was that they were caused by gods or dragons. Today, we know the cause is bioluminescence.
The writings of the ancient Eastern civilisations are the earliest known recordings of bioluminescence. These contained information on the firefly and the glow-worm. The first mentions of these attributes of bioluminescent organisms came from the Greeks and Romans. Additionally, 180 marine species were described by Aristotle (384-322 BC). In 1492, Christopher Columbus came across ‘mysterious lights in the water’ and was unsure of the cause.
Robert Boyle recorded in 1667 that the condition for luminescence is air. We are now aware that it is not just air that is required, but more specifically oxygen, as this is involved in the oxidation of luciferins. In 1956, the first luciferin was isolated by Green and McElroy, providing vital evidence for our understanding of bioluminescence today.
|

Bioluminescent glow worms in Waitomo Caves, New Zealand.
Photo: © E. Wigzell and T. White, used with permission. |
So, how does bioluminescence work?
Luciferins can be excited (well I’d be excited if I was a molecule which could glow!) by undergoing an oxidation reaction that is catalysed by enzymes. This means that an electron moves up to a higher energy level. When the luciferins then relax back to their ground state, they release photons of light, causing bioluminescence.
However, the exact mechanism varies for different types of luciferin. Oxidation of luciferin can sometimes occur in aqueous solution, in the presence of an enzyme (luciferase) and dissolved oxygen. This has been shown for several groups of animals.
Let’s hear some chemistry, then...
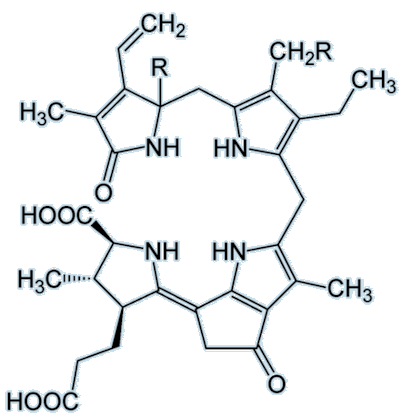
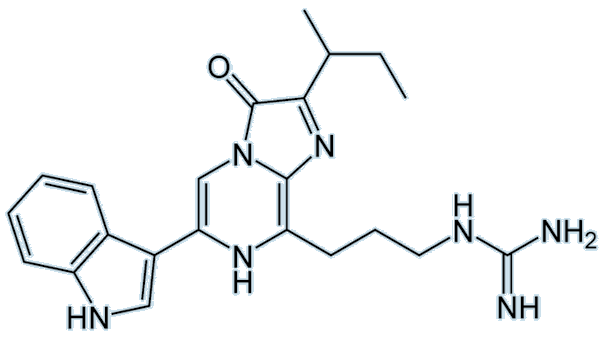
The name luciferin refers to a group of compounds that, when oxidised in the presence of an enzyme (luciferase), produce visible light. Each luciferin has its own luciferase — a specific enzyme which catalyses the reaction. The chemical structures vary with each compound, for example:
 |
 |
| Dinoflagellate luciferin |
Vargulin luciferin |
As you can see, these two luciferins look completely different to one another. The vargulin luciferin has a much smaller structure than dinoflagellate luciferin and has no –COOH group. The dinoflagellate luciferin also has five rings compared to three on the vargulin luciferin. A common luciferin to focus on and marvel at is the firefly luciferin:
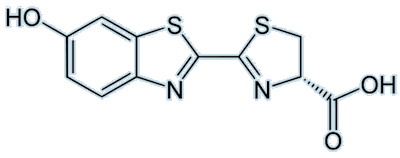 |
| Firefly luciferin |
I love a good structure... but how does the whole thing work?
Firefly luciferin (or (4S)-2-(6-hydroxy-1,3-benzothiazol-2-yl)-4,5-dihydrothiazole-4-carboxylic acid, if you prefer) is, of course, the luciferin responsible for fireflies emitting beautiful light. Visible emission occurs when oxyluciferin relaxes from an excited state to the ground state. The colour emitted differs, even if the luciferin used is the same; this may be due to changes in pH or differences in the structure of the luciferase involved.
The reaction in fireflies involves the oxidation of the luciferin using various enzymes and reagents such as ATP (see MOTM for Jan 1998). This results in removal of an H atom from the ring at the position marked by a red 4 in the diagram, below. The molecule then rearranges to form a peroxy compound called 1,2-dioxetane (compound 4 in the figure below). This compound is unstable so decays spontaneously to CO2 and an excited ketone (5) (yes, it’s all very exhilarating!). The energy is released by de-excitation which emits visible light.
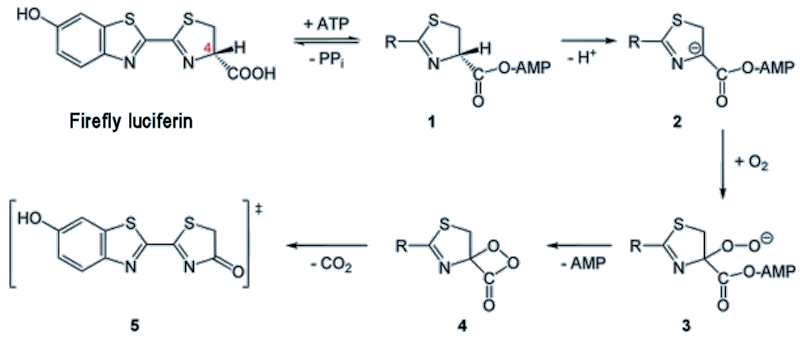
Mechanism for firefly luciferin reaction. ATP = adenosine triphosphate, AMP = adenosine monophosphate, PP = pyrophosphate.
Electronic excitation is denoted by the double-dagger ‡ symbol, and this molecule will lose this excess energy by emitting light.
Okay, so glowing in the dark is pretty awesome, but biologically, is there a point?
|
There are many theories regarding the biological advantages of bioluminescence from the point of view of survival and reproduction. Scientists believe that many organisms use this colourful and luminous display of light as a form of camouflage (as counterintuitive as it may seem). An example of this is the Hawaiian bobtail squid (shown right), which benefits from mutualistic bioluminescent bacteria living in an organ in its mantle. The bacterial light helps to camouflage the squid against moonlight and remove their shadow, hence protecting it from predation.
Another useful advantage of bioluminescence is to attract prey. Glow worms (Arachnocampa flava) in their larval stage, and several species of fungus gnats, use silk covered in glowing mucous to attract and trap unsuspecting airborne insects (it’s practically breakfast in bed!). Many other organisms flash in response to a threat or being disturbed. Dinoflagellates, a type of plankton, are an example. These unicellular creatures light up the sea with a bluish glow when disturbed, again, all thanks to the presence of a luciferin.
|
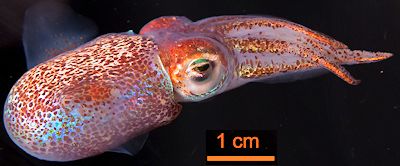
Adult Hawaiian bobtail squid (Euprymna scolopes).
Photo: M. McFall-Ngai in PLoS Biol 12 (2014) e1001783 via CC BY-SA 4.0 licence.
|

Fireflies in a tree.
Photo: Sushantk2212 in Wikimedia Commons via CC BY 4.0 licence.
|
Luciferin and the ‘love bug’
A common symptom of falling in love in humans is often described as ‘glowing’, however, this is actually the case for fireflies, which literally ‘light up each other’s worlds'. Male fireflies attract females by emitting a beautiful pattern of visual signals - bit like an insect Morse code. While many organisms use bioluminescence caused by luciferins to attract prey or mates, fireflies also use the properties of these compounds to discourage predators from attempting to eat them; the glow warns potential predators of the toxic lucibufagins which the insects contain.
|
Is it useful for humans, or is it just something pretty for us to look at?
Luciferin actually has many applications within the medical field. One of the main ones is bioluminescent imaging, which can be used in vivo (inside a living organism) for whole-body imaging. Recently, this was used in monitoring the growth of brain tumours in mice. The gene that encodes for firefly luciferase is introduced as a reporter gene into the mouse and the photons which are released when the luciferase oxidises the luciferin are detected using bioluminescent imaging (see photo, right). This allows scientists to monitor the growth of the brain tumour, and the response of the tumour to treatment. Bioluminescent imaging is highly sensitive, so it can detect very small concentrations. This, coupled with the fact that firefly luciferase has a fast turnover, means real-time measurements can be made.
|
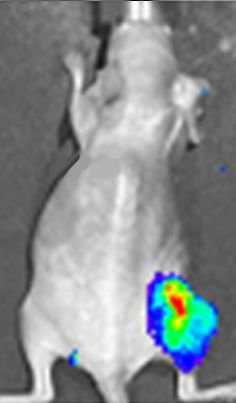
Luciferase expression in a mouse tumour shown in a bioluminescent image.
Photo: D. Haddad et al. in Plos One 7 (2012) e41647 via CC BY 4.0 licence.
|
I know where this leads: ‘Click’ chemistry
Yes, ‘click’ chemistry is a group of reactions which allow chemists to attach a probe to a specific biomolecule. We can use the principles of bio-orthogonal chemistry (chemical reactions which can occur within a living system without having an impact on the biochemical processes natively occurring) and ‘click’ chemistry to create functional luciferin probes to be used inside cells. This can be used to get luciferin into a cell whilst attached to another molecule. For example, when recording the uptake of palmitate by an imaged brown adipose tissue, chemists can join the palmitate to the luciferin, with the palmitate acting as a cage preventing the luciferin from being excited. The luciferin remains inert until it enters the cell, at which point the bonds caging it break, and the molecule becomes excited, releasing light which can be recorded using the imaging technology. In this reaction, the amount of light is proportional to the uptake of palmitate.
So this ancient biological mystery can be explained by a bit of chemistry?
Yes, good old molecules (but it also needed a bit of physics and biology to discover the full story, don’t forget!). It even turns out that luciferin bioluminescence isn’t just easy on the eyes, its potential medical applications are pretty fab as well.

Bibliography
Bioluminescence and History
Chemical Structure and Reactions
Organisms
Medical Applications
- Genevois, C., Loiseau, H., Couillaud, F. In Vivo follow-up of brain tumour growth via bioluminescence imaging and fluorescence tomography, Int. J. Mol. Sci. 17, (2016) 1815.
- Momcilovic, M. and Shackelford, D. Imaging Cancer Metabolism, Biomolecules and Therapeutics 26,(2017) 81-92.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.9988682]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.9988682]
![]()
![]()
![]()
![]()










