![]()
Lycopene
The red colour of tomatoes
![]()
![]()
Molecule of the Month January 2020
Also available: JSMol version.
![]()

LycopeneThe red colour of tomatoes
Molecule of the Month January 2020
|
 |
Yes, it’s due to lycopene, a long-chained hydrocarbon that’s present in tomatoes, especially in the skin. Lycopene is a natural colouring agent, and is bright red.
 Is it responsible for the colour of all red fruits and vegetables, too?
Is it responsible for the colour of all red fruits and vegetables, too?No, although it is found in some other red fruits like watermelons, papayas, pink grapefruit, pink guava, goji berries and rosehips, as well as in red carrots (although most of their orange colour comes from a related molecule, β-carotene [see MOTM for April 2002]). Indeed, one of the best sources of lycopene is actually tomato ketchup sauce! However, lycopene is not present in other red fruits such as strawberries and cherries.
The structure of the molecule gives a clue. Lycopene consists of a long chain of conjugated double bonds, i.e. double bonds that alternate with single bonds along the molecule. The p-orbitals from each of these carbons overlap with those of their neighbours to effectively form one long molecular orbital that stretches the length of the molecule. Electrons can move freely along this orbital, but cannot escape it, so they behave like ‘particles in a 1-dimension box’ (the orbital has a significant length, but very little width or height). Applying the rules of quantum mechanics to this 1-D box reveals that the electrons, behaving as waves, can only adopt a certain set of stable vibrational frequencies with fixed energies. As such, the electrons can only absorb certain wavelengths of light; the wavelengths of light that are not absorbed are reflected back to the observer and give the molecule its apparent colour.
 |
| Lycopene |
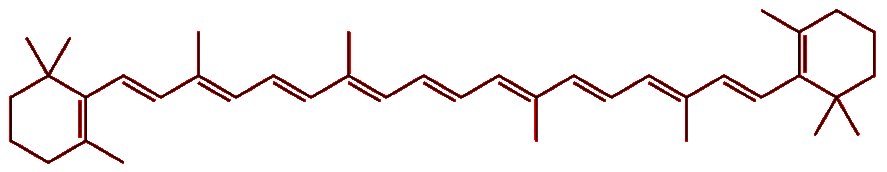 |
| β-Carotene |
|
In the case of lycopene, the high-energy blue and green wavelengths are absorbed, while the lower energy red light is reflected, and so it appears red. For β-carotene the absorption is shifted slightly more to the blue end of the spectrum, and so the reflected light appears more yellowy-orange. This is because, although β-carotene has 11 conjugated double-bonds just like lycopene, the slight differences in the shape of the two molecules subtly change their energy levels, and with it, their absorption spectra. |
 But why does tomato juice stain so badly?
But why does tomato juice stain so badly?Again, the structure of the molecule gives a clue. Lycopene only contains hydrogen and carbon, so does not have any hydrophilic groups like carbonyls or hydroxyls. That means that lycopene does not dissolve in water, but only in oils or non-polar solvents. Because of this, washing tomato-stained clothes or porous cookware with hot water will not dissolve the stain, and even some soaps are not strong enough to extract the lycopene from its strong attachment to the substrate. However, dilute bleach (MOTM for October 2011) works, because it attacks and oxidises the double bonds which can then allow the molecule to dissolve in water.
Because of its structure, lycopene is believed to act as an antioxidant, removing harmful free radicals in the body. As a result, there are a host of ‘health food’ shops and web sites that claim all sorts of medical benefits from eating lycopene-rich foods or supplements. These promised benefits include sun protection, improved heart health and a lower risk of certain types of cancer. Although there are a couple of studies that show that lycopene consumption may reduce the risk of heart disease and some cancers, the general view of both the US FDA and the FSA in Europe is that there is not sufficient evidence yet to show that lycopene has any real beneficial effects. But, of course, the facts don't stop the marketing people from exaggerating, or even inventing, the 'benefits' that may be obtained if you buy their lycopene supplement. On a positive note, lycopene is also not considered harmful in normal amounts – and is often used as a red food-colouring agent (in the EU it has the E-number: E160d).
 So why are tomatoes red?
So why are tomatoes red?To attract birds and animals to eat them. Tomatoes contain internal seeds which when eaten pass through the animal’s digestive system unharmed and are then excreted some distance away, maybe many miles in the case of birds, from the original plant. This helps to propagate the tomato plant and allow them to spread further afield.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.8319998]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.8319998]