![]()
Semaglutide
The diabetes drug that might also be a cure for obesity
![]()
Paul May
University of Bristol
![]()
Molecule of the Month - May 2021
Also available: HTML version.
![]()

[Photo licensed under CC BY-SA]
|
SemaglutideThe diabetes drug that might also be a cure for obesity
Paul May
Molecule of the Month - May 2021
|
 [Photo licensed under CC BY-SA] |
Yes, a drug that can treat both diabetes and help you lose weight may save thousands of lives.
Well, as you may already know, diabetes is a metabolic disorder characterized by a high blood-sugar level (hyperglycaemia). Sugar (glucose) is naturally present in the bloodstream as a result of digestion of foods, but in order for the sugar to be stored in various organs (predominantly the liver) for later conversion into energy, it requires the presence of the hormone insulin (see MOTM for July 2010). Insulin is produced in the pancreas, and more insulin is produced if glucose levels are high to mop up the excess sugar, while less insulin is produced when glucose levels are low. The secretion of insulin into the blood in response to the concentration of glucose maintains our glucose levels within safe limits.
Diabetes is caused either by the pancreas not producing enough insulin, or the cells of the body not responding properly to the insulin produced. There are 2 main types. In Type 1 diabetes (also known as juvenile diabetes), the pancreatic cells that produce insulin are destroyed so that insulin can no longer be synthesized or secreted into the blood. Type 2 diabetes (adult-onset diabetes) makes up about 90% of all diabetes cases and occurs when the pancreatic cells are damaged or disrupted. Insulin is still secreted, but in insufficient amounts to properly regulate the blood glucose levels. Although some people are genetically more susceptible than others, there is now a lot of evidence to suggest that Type 2 diabetes occurs primarily due to obesity and lack of exercise.
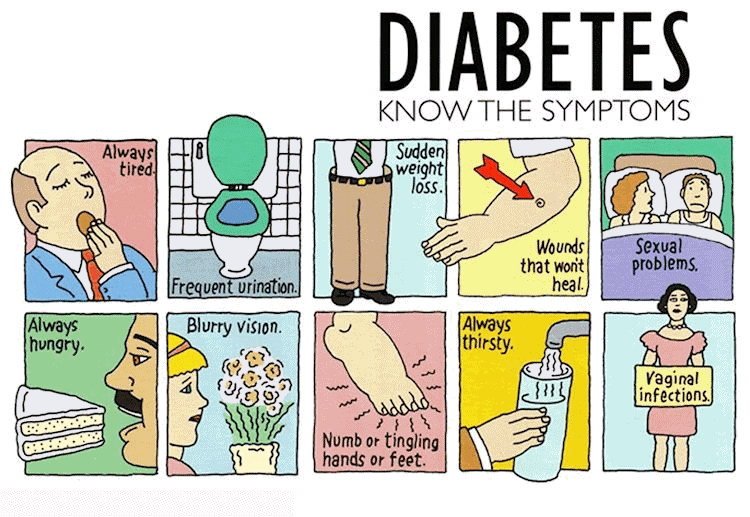
[Author: Unknown. Licensed under CC BY-NC-ND]
Over a period of time, people with high blood sugar can develop serious or life-threatening problems, including heart disease, stroke, kidney problems, nerve damage, and blindness.
How is it treated?Type 1 diabetes is incurable, but it can be treated using regular injections of insulin. The person’s blood-sugar levels must be measured several times a day, especially after eating, usually by pricking their finger and testing the blood drop. If the glucose levels are too low, they need an injection of insulin. However, the dosage is easy to get wrong, and if too much insulin is injected the blood-sugar drops too low (hypoglycemia) and the person can feel faint or even pass out. Often simply giving the person a sweet drink or sugary biscuit is enough to bring their blood sugar back to normal values. |
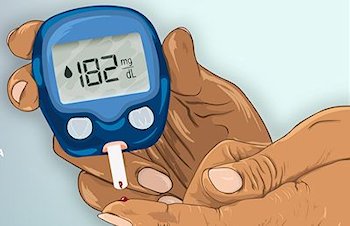 Testing for blood sugar levels. [Photo: Unknown author, licensed under CC BY-SA.] |
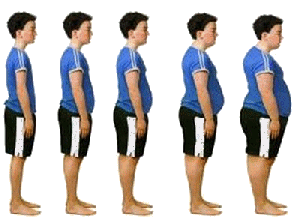 Childhood obesity has tripled in the last thirty years, and two-thirds of America is now classed as overweight or obese. [Photo: Unknown Author is licensed under CC BY-ND] |
In contrast, Type 2 diabetes is largely preventable by not becoming obese, doing regular exercise, and eating a healthy balanced diet that doesn’t contain excessive sugar. It can be treated by simply making lifestyle changes (e.g. improved diet, more exercise), and some medication (e.g. metformin), but some people still require insulin injections. Since 1960, the rates of Type 2 diabetes have risen hugely, along with an increase in obesity levels, such that now it is becoming a major health issue in developed countries. |
Precisely. For Type 2 patients, one answer is to search for a drug that controls blood-sugar levels in a similar way to insulin, but is more convenient to use.
Luckily scientists had a clue, because they already knew about some likely molecules based on decades of studies of digestion. In response to eating food, the intestine produces a group of very similar hormones called incretins, which travel in the bloodstream and act on the pancreas to increase insulin secretion. The most important of these incretins is called Glucagon-Like Peptide 1 (or GLP-1), and as well as encouraging the release of insulin from the pancreas, GLP-1 also increases the volume of cells in the pancreas that produce insulin. Thus, more GLP-1 leads to more insulin, which decreases blood-sugar levels.
So you can just give diabetics GLP-1?Unfortunately, not. In the body, GLP-1 is rapidly degraded within about 2 minutes. So, a diabetic person would need to inject themselves with GLP-1 every couple of minutes to properly control their blood-sugar levels – which obviously isn’t possible So what did they do?One solution was to replace GLP-1 with another protein which has a very similar structure (a GLP-1 analogue), but which has a longer lifetime in the body. Various proteins were tried, including one obtained from the venom of a Heloderma lizard(!), with varying degrees of success. A different approach, however, involved using a taxi. |
 A Gila monster (Heloderma Lizard). [Photo: Modified from MonsterDoc, CC BY-SA 4.0 via Wikimedia Commons.] |
What do you mean, a taxi?Well, a taxi-molecule to be precise. This is a molecule that can transport the drug molecule (in this case GLP-1) around the body in the bloodstream, and deliver it to its destination – just like a taxi. The taxi molecule prevents the GLP-1 from degrading during its journey, and thus extends its lifetime considerably. What was this taxi-molecule?Again, this was chosen based on prior knowledge of the body’s biochemistry. Human serum albumin (HSA) is a heart-shaped protein that is naturally produced daily in small amounts by the liver. Its plays many roles in the body, helping to keep the body’s chemistry and fluid balance within pre-set limits (homeostasis). It helps stabilise the pH of blood plasma and it is also an important antioxidant. |
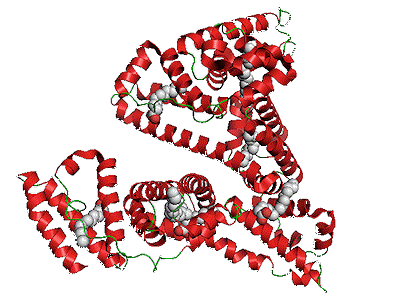 Structure of HSA containing 565 amino acids shown in ribbon form. [Photo: Public domain, via Wikimedia Commons] |
There are three reasons that it makes a good taxi molecule. It is a naturally occurring protein, so is not toxic in small amounts. It is particularly stable in the bloodstream – a taxi would be no good if it broke down or disintegrated before the passenger arrived at their destination. And it has a large number of binding sites that allow proteins, amino acids and many other reactive molecules to attach to it in multiple different ways – like a taxi being able to hold passengers in the front seat, back seat, in the boot (trunk) and on the roof-rack! Because HSA can transport otherwise insoluble molecules, such as fatty acids and steroids, around the body, scientists have often used it as a conduit for numerous water-insoluble drug-molecules which need to travel through the bloodstream.
Unfortunately, it wasn’t quite as easy as that. The two protein molecules are not the right shape to bond to each other, and so a 'linker molecule' is required that joins the two together. Rather than using the taxi analogy, a better one now would be one car towing a caravan via a tow-rope. In this case the car is HSA, the caravan is GLP-1, and the linker is chain of several γ-glutamate molecules which as the glue with which to attach a long-chained fatty acid tail (see MOTM for May 2006) which acts as a spacer.
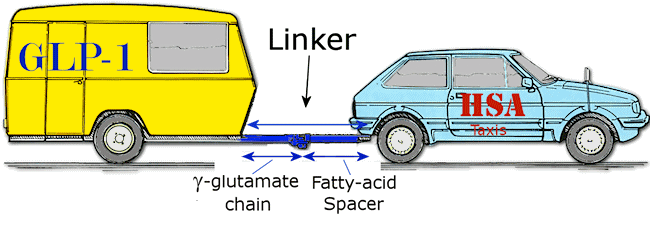
In theory, yes. But in practice the scientists had to test out a number of different linker molecules, and chemically modify the GLP-1 structure slightly, in order to get the new drug to work.
The scientists found that with linkers that were too short, the GLP-1 would either not bind the HSA strongly enough and it would have a very short lifetime in the body, or alternatively, if they were too long, the HSA would bind too strongly so that it wouldn’t be released when needed. Going back to the taxi analogy, it would be as if the taxi ejected the passenger before it got to the destination, or when it arrives there, the door was jammed locking the passenger inside! Having a spacer with a chain length of about 16 carbons was the right compromise; giving the drug a lifetime in the bloodstream of hours meant that the patient could have the treatment – usually as an injection - daily.
Yes – the first such drug, liraglutide, was marketed by the drug company Novo Nordisk under the names Victoza or Saxenda, and was approved for medical use in Europe in 2009 and in the United States in 2010. In 2017, it was the 163rd most commonly prescribed medication in the USA, with more than 3 million prescriptions.
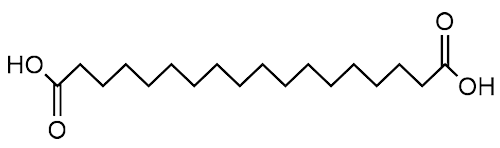
Daily injections are still rather inconvenient, so scientists continued modifying the chemistry to try to increase the lifetime of the drug in the body, ideally to allow a weekly rather than daily injection. As well as modifying the protein further, they found that if they used a long-chained fatty bi-acid (i.e. one with a carboxylic acid group at either end of the carbon chain) with 18 carbons in the chain (stearic bi-acid), they achieved their aim.
 |
|
| Stearic bi-acid |
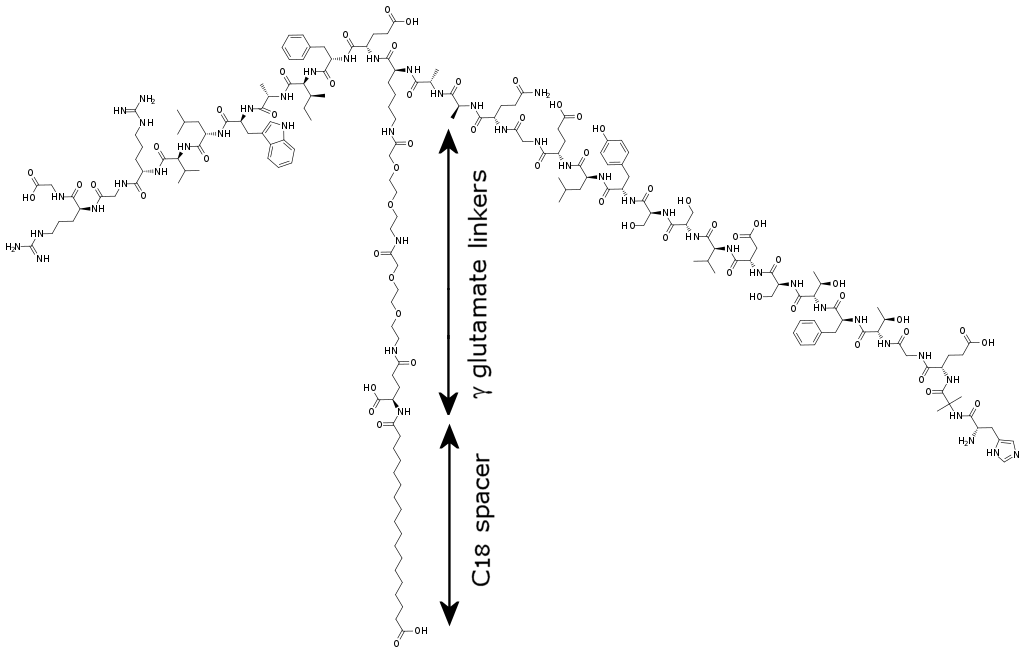
The resulting drug was called semaglutide, and has been sold under the brand name Ozempic since 2017. A version that could be taken orally as a pill, rather than injection, has just become been available (since 2019) under the name Rybelsus.
 |
|
| Structure of semaglutide, showing the GLP-1 protein at the top, and the γ-glutamate linkers joined to the C18 stearic biacid spacer. The HSA (not shown) would attach to the carboxylic acid group at the bottom of the C18 chain. [Image: Ed (Edgar181), Public domain, via Wikimedia Commons] |
As well as increasing insulin secretion, GLP-1 also increases the feeling of fullness during and between meals by acting on appetite centres in the brain and by slowing ‘gastric emptying’ - which is a fancy way of saying it slows down the process by which the stomach sends its partly digested contents down into the intestine. This means the person stays fuller for longer, and has no inclination to eat more food. In other words, it acts as an appetite suppressor.
Semaglutide is now available in various forms.
A number of studies have shown this, and a recent (2020) international trial of 2000 people (see R.J.H Wilding, 2020 reference, below) has shown that weekly injection of semaglutide (together with advice on diet and fitness) gave an average weight-loss of 15 kg during the 15-month trial . Some people even lost up to 1/5th of their body weight! Many medics are saying that this could be a ‘game-changing’ treatment for obesity.
 Wow!
Wow!But there are still more trials to be done, especially on larger samples over longer time periods. And there are some worries about side-effects (nausea, diarrhoea, vomiting, and constipation), and safety concerns (as with any new drug) which need to be dealt with before it becomes certified for use. There are also questions as to what happens to the patients once they stop taking the drug. Some have reported losing the weight while on the drug was much easier than before, but as soon as the trial stopped their appetite returned and their weight started to increase again. So maybe the drug needs to be used in combination with a sensible diet and exercise regime, if the weight is to stay off.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.13992209]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.13992209]