
|
UBIQUITIN |

|
Structure |
|
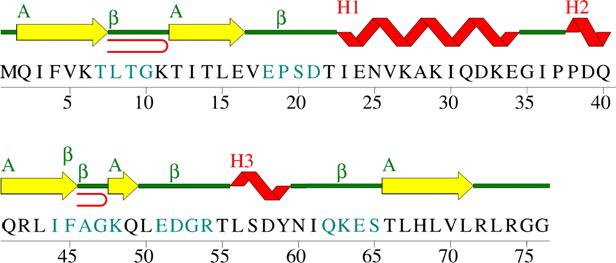
Secondary structure: (This is the local folding pattern of polypeptide chain) Figure 1. Wiring diagram of secondary structures for human ubiquitin (1ubq). Yellow are β-strands; Red are helices; Seablue characters are amino acids that make up β-turns; Red curves correspond to β-hairpins [33]. Sumary of secondary motifs present in human ubiquitin [33]: a). 1 β-sheet: - one sheet (A) consists of 5 β-strands; type: mixed; no barrel; topology[?]: -1 3X 1 –2X. b). 5 β-strands (31,6%): c). 3 Helices (15,8% of a-helix; 7,9% of 3,10 helix): d). 6 β-turns: e). 1 β-bulge: - type: G1; antiparallel; residue X: T7; residue 1: G10; residue 2: K11. f). 2 β-hairpins: |






|
Number of stand |
Start |
End |
Belongs to sheet |
Edge |
Number of residues |
Sequence |
|
1 |
2 |
7 |
A |
- |
6 |
QIFVKT |
|
2 |
12 |
16 |
A |
+ |
5 |
TITLE |
|
3 |
41 |
45 |
A |
- |
5 |
QRLIF |
|
4 |
48 |
49 |
A |
+ |
2 |
KQ |
|
5 |
66 |
71 |
A |
- |
6 |
TLHLVL |
|
Helix no. |
Start |
End |
Type |
No. of residues |
Lenght [Ǻ] |
Unit rise |
Residues per turn |
Pitch [Ǻ] |
Delia-tion [degrees] |
Sequence |
|
1 |
23 |
34 |
H |
12 |
17,52 |
1,46 |
3,68 |
5,38 |
10,8 |
IENVKAKIQDKE |
|
2 |
38 |
40 |
G |
3 |
- |
- |
- |
- |
- |
PDQ |
|
3 |
56 |
59 |
G |
4 |
6,77 |
1,69 |
3,45 |
5,82 |
40,1 |
LSDY |
|
No. of turns |
Start |
End |
Turn type |
H - bond |
Sequence |
|
1 |
7 |
10 |
I |
+ |
TLTG |
|
2 |
18 |
21 |
I |
+ |
EPSD |
|
3 |
44 |
47 |
IV |
- |
IFAG |
|
4 |
45 |
48 |
I’ |
+ |
FAGK |
|
5 |
51 |
54 |
I |
+ |
EDGR |
|
6 |
62 |
65 |
II |
+ |
QEKS |
|
Strand 1 |
Strand 2 |
Class |
||||
|
Start |
End |
Lenght |
Start |
End |
Lenght |
|
|
2 |
7 |
8 |
12 |
16 |
5 |
3:5 |
|
41 |
45 |
5 |
48 |
49 |
2 |
2:2 |