The Variational Method:
This is a method, based on the variational principle, for
finding approximate wavefunctions. It
is our main weapon in the struggle to find electronic wavefunctions for
molecules and enables us to calculate molecular orbitals, molecular electronic
wavefunctions and potential energy curves and surfaces. The method works as follows:
1. Construct an approximate wavefunction y which depends
on some parameters ci.
2. Calculate the energy using equation (1). The energy calculated in this way, E(ci),
will depend on the parameters ci
which are part of the approximate wavefunction.
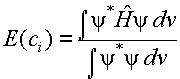 (2)
(2)
3. Choose
the parameters ci so as to minimize the energy
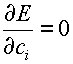 (3)
(3)
· This minimized
energy is still above the true ground state energy.
· The
wavefunction with this set of parameters is the best wavefunction of the form
which has been chosen.
·
It gives the lowest possible energy, as close as possible to
that of the exact ground state wavefunction.