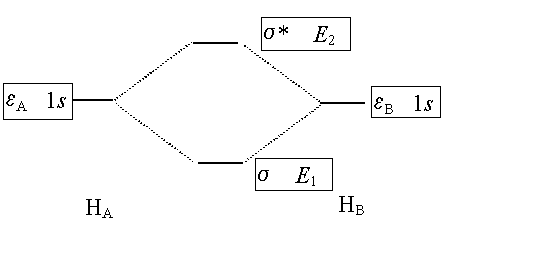
The Non-Crossing
Rule:
When we “mix” two wavefunctions to form a “better”
wavefunction the new energies “repel” each other. The lower of the two new energies is lower than either of
the original two energies while the higher one is higher than either of the
original energies. A familiar
example is the mixing of the two 1s atomic orbitals of the hydrogen
atoms in H2. They “mix”
to form bonding and antibonding molecular orbitals: