The Hückel approximations applied to ethene
Making
approximation (iii) and taking f1 and f2 to be normalized (i.e., that S11 and S22
both equal 1) the secular equations in (2) become
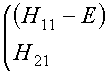
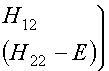
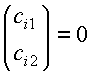 (4)
(4)
Simplification of the Hückel molecular
orbital (HMO) matrices:
The secular equation for
ethene, once the Hückel approximations have been applied, should have the form:
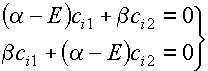
To give this
equation a simpler form we divide through each line by b and set
x=(a – E)/b to obtain:
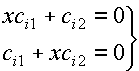
or
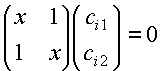 (6)
(6)
The energies
are now measured in units of b (which you
should remember is a negative number!)