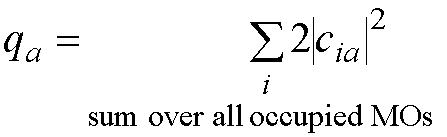p electron atomic charge
The coefficients in the molecular
orbitals reflect the charge distribution of an electron in that orbital.
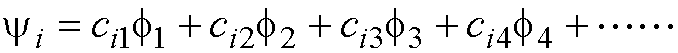
yi: Molecular
orbital number ‘i’
f1: atomic (p)
orbital on atom 1
f1: atomic (p)
orbital on atom 1, etc.
In the coefficients cia
i ----- is the molecular
orbital number
a ----- is the number of the atom and of
corresponding atomic p-p orbital.
If there is an electron (just 1) in
molecular orbital yi the charge
distribution corresponding to this electron is given by:
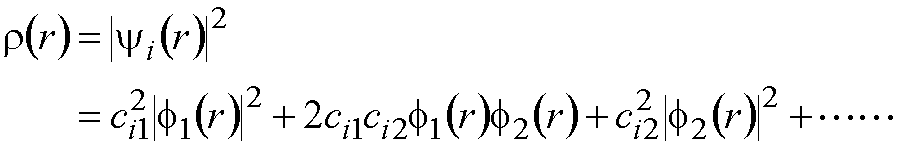
r(r): Charge
distribution (electron density) -- this is a function of position r --
arises from 1 electron in M.O. “i”
The electron density is a function of the coordinates. Its value changes with position in space.
In the example above, ci12 is
a measure of the amount of charge on atom 1 - arising from 1 electron in
MO i.
2ci1ci2 is a measure
of the charge shared between atoms 1 and 2.
p electron charge on atom a (qa):
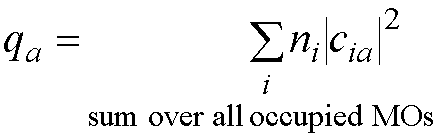
ni is the number
of electrons in MO i.
If the occupied molecular orbitals all
have 2 electrons in them then ni=2 and the equation for the
atomic charge becomes