So we can calculate the activation free energy D‡G, activation enthalpy D‡H and
activation entropy D‡S, broken down
into rotational, vibrational, translational and electronic terms.
This gives insight into which factors are most important in determining the barrier to a reaction.
For example, for the Diels-Alder reaction:
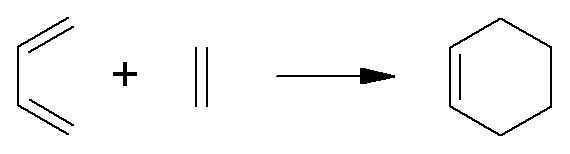
|
|
D‡H |
–TD‡S |
|
electronic |
74.9 |
0 |
|
vibrational |
14.2 |
–6.7 |
|
rotational |
–3.8 |
14.2 |
|
translational |
–6.3 |
43.5 |
|
total |
79.0 |
51.0 |
(in kJ mol–1, at 300K).
For the SN2 reaction of OH– with CH3F
to form CH3OH and F–:
|
|
D‡H |
–TD‡S |
|
electronic |
21.3 |
0 |
|
vibrational |
8.8 |
–8.8 |
|
rotational |
–2.5 |
0.4 |
|
translational |
–6.3 |
33.9 |
|
total |
21.3 |
25.5 |
(in kJ mol–1, at 300K)
For the Claisen rearrangement of allyl vinyl ether to form 5-hexenal:
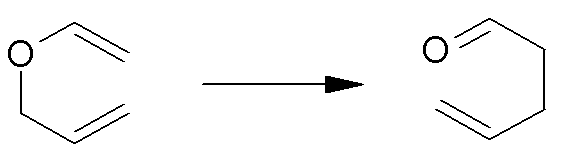
|
|
D‡H |
–T‡DS |
|
electronic |
97.9 |
0 |
|
vibrational |
–5.4 |
10.9 |
|
rotational |
0 |
0.4 |
|
translational |
0 |
0 |
|
total |
92.5 |
11.3 |
(in kJ mol–1, at 300K)
· All these
calculations used the MP2 method (e.g. with the 6-31G(d) basis set).
· In all these
cases, the vibrational energy contribution includes the difference in
zero-point energy between the reactants and the products.
· All of these
contributions to the energy barrier can be found from the structure and energy
of the reactants and TS, and from the partition functions.
· For example, the
vibrational contribution can be found from the calculated harmonic frequencies
(the TS has one less frequency).
· The energy
gaps between translational levels, and between rotational levels, are very
small, which means that these contributions can be calculated classically (e.g.
each degree of freedom contributes RT/2
to the enthalpy). Remember that in a
reaction like this, two molecules are coming together to form a single TS, so 3 rotational
degrees of freedom and 3 translational degrees of freedom are lost going to the
TS (for
non-linear reactants). There is also a PV correction to the translational term,
on going from 2 moles (reactants) to one mole (TS), i.e. PDV = DnRT = –RT (Dn = –1). Altogether this means that D‡Htrans and DHrot contribute –4RT per mole to D‡H for a bimolecular reaction (of two non-linear molecules). The translational and rotational entropy
changes are large and negative, opposing formation of the TS.
· For a
unimolecular reaction, translation and rotation make no contribution to D‡H (the number of rotational and translational modes, and the
number of moles, is the same as for the reactants), and the entropy changes are
small
· For all
reactions, the vibrational contribution may be significant. The largest contribution to the
barrier, however, is often the electronic energy difference between the
reactants and the TS. This energy is
the energy of the electrons and nuclei in the molecule, with the nuclei at
rest. That is, the geometry of the
molecule is fixed (at the minimum or TS structure). The electronic energy is the energy which is plotted on a
potential energy surface diagram.