
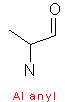
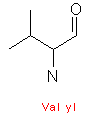
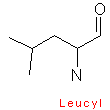
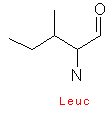
Amino acids are designated by a three letter abbreviation, or a one letter symbol. The exceptions are tryptophan (Trp), asparagine (Asn), glutamine (Gln), and isoleucine (Ile). Small amino acids are denoted by the first letters of their names. This vocabulary is imperative, as it is an integral part for every biochemist.
Here is a list of some of the principle amino acids:






Amino acids have aliphatic side chains. The simplest amino acid is glycine, which has just one hydrogen atom as its side chain. Alanine comes next with a methyl group as its side chain. Valine, leucine, and isoleucine have larger hydro-carbon side chains, and these chains are said to be hydrophobic, and hence remains in its cluster form.
Proline
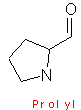 also has an aliphatic side chain, but is
considerable different to the other amino acids, in a way that it has its side
chain chain bounded to both the nitrogen and to the alpha - carbon atoms.
also has an aliphatic side chain, but is
considerable different to the other amino acids, in a way that it has its side
chain chain bounded to both the nitrogen and to the alpha - carbon atoms.
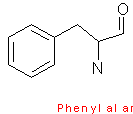
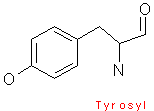
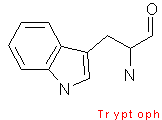
Phenylalanine contains a phenyl ring attached to a methylene group. Tryptophan has an indole ring attached to the methyl group. These two amino acids are very hydrophobic. Tyrosine is less hydrophobic due to the attached hydroxyl group which is very reactive.
The aromaticity of phenylalanine, tryptophan, and tyrosine all contain delocalized pi-electrons, and are able to react with other pi-systems, and can hence allow easy electron transfer.
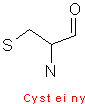
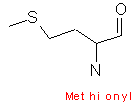
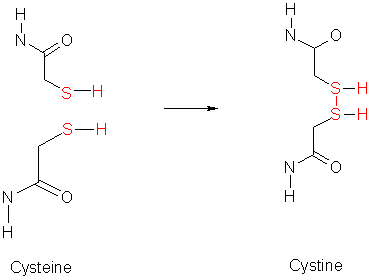
Cysteine contains a sulphydryl group (-SH), and can form disulfide links. Methionine contains a sulphur atom (-S-CH3). Both of these sulfur containing reagents are hydrophobic. The sulfide group on cysteine is very reactive, as it is important in the formation of disulfide bonds, which are formed by oxidation of cysteine residues. The resulting product is called cystine, this is illustrated by;
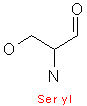
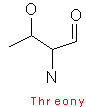
Serine and threonine both contain hydroxyl groups, which make it very hydrophilic, and hence more reactive than say alanine or valine. Threonine is optically active, as it has two centers of symmetry, glycine is optically inactive, and all other amino acids, except for glycine have an asymmetric alpha - carbon center.
The uniqueness of histidine is that depending on its environment, it can be positively charged or uncharged, and is a major amino acid to be found in the active site of enzymes. The reaction is the imidazole ring, which readily catalyzes the reaction by the making and breaking of bonds. It is important to note that lysine, arginine, and histidine are basic amino acids . Acidic amino acids are for example aspartate and glutamate, which have negatively charged oxygen atoms, (making it a good nucleophile).
Amino acids are linked by peptide bonds to form polypeptide links.