![]()
![]()
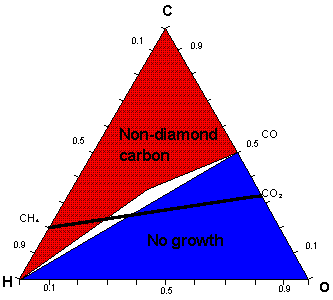
Low-pressure diamond deposition has been achieved using a large range of C, H and O containing gas mixtures. Bachmann et al summarized the results of many deposition experiments involving various gas mixtures and reactor types in the form of an atomic C-H-O phase diagram (Fig. 1). They concluded that the exact nature of the source gases was unimportant for most diamond chemical vapor deposition (CVD) processes and that, at typical process temperatures and pressures, it was only the relative ratios of C, H and O that controlled deposition.
The diagram partitions into three distinct regions associated with: (a) diamond growth, centered on the C-O tie line where the input mole fractions of carbon and oxygen are equal (i.e. [C] = [O]); (b) no growth, lying below the C-O tie line (i.e. [C] < [O]); and (c) non-diamond growth, located above the C-O tie line (i.e. [C] > [O]).
Previous work within our group, concerning diamond growth from CO2/CH4 gas mixtures, has confirmed the presence of three such regions. This work also involved the modeling of gas-phase chemistry for the entire range of CO2/CH4, using the CHEMKIN II computer package. Figure 1 shows a schematic of the Bachmann C-H-O atomic phase diagram with the full range of CO2/CH4 gas mixtures shown as a line cutting the diagram.
The simulations were run for the full range of each gas mixture using the following conditions:
| Reaction scheme | All relevant C-H-O containing reactions from the GRI-Mech 2.11gas-phase reaction kinetic database |
| Gas temperature | 2000 K |
| Pressure | 40 Torr |
| Reaction Time | 5 s |
 | 
|
 | 
|
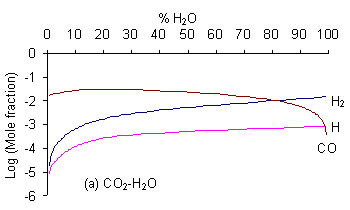
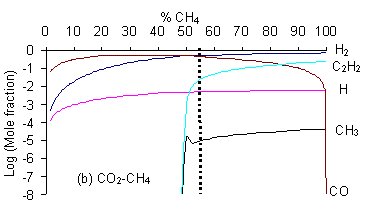
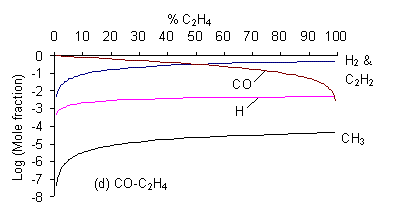
It can be seen that for the CO2/H2O mixture {which lies in the no growth region of the C-H-O diagram) both [CH3] and [C2H2] are negligible (<10-10 whereas for a gas mixture lying within the non-diamond growth region (i.e. CO/C2H4) the mole fractions of both these species are considerably higher (~ 10-6 and 10-2 respectively). It should be noted that there is a jump of several orders of magnitudein both [CH3] and [C2H2] for all mixtures when crossing the H-CO tie line (i.e. CO2/CH4). CH3 is beleaved to be the species responsible for diamond growth as is confirmed by this result as this line also markes the boundary between the non growth and diamond growth domains (as shown below in Fig. 2).
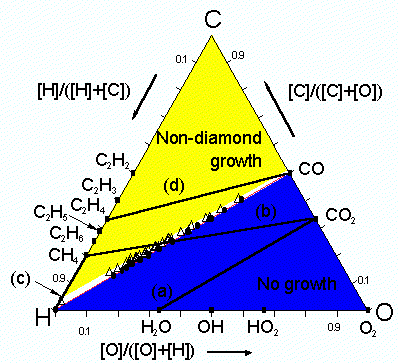
The lower boundary of the diamond growth domain (i.e. the no growth-diamond growth boundary) of the C-H-O atomic phase diagram can therefore be defined in terms of [CH3]. It should also be possible to define the upper (diamond-non diamond) boundary in a similar manner. Diamond growth is generally believed to be the result of simultaneous deposition of diamond and graphitic phases with the graphite being etched by atomic H atoms to leave diamond. C2H2 is linked to the deposition of graphitic films therefore the diamond-non diamond growth boundary can be deffined in terms of the ratio [H]/[C2H2]. The value of 0.2 for this ratio gave a boundary in good agreement with experimental results (Table. 2) allowing the regions of the C-H-O atomic phase diagram to be predicted as shown above in Figure. 2.
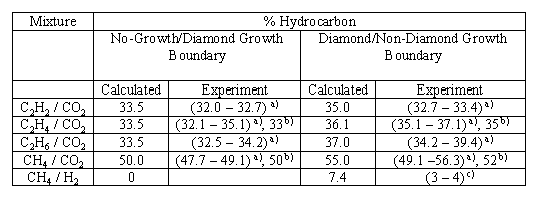
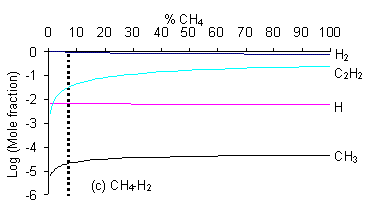
we note that the present simulations predict the diamond to non-diamond growth boundary for CH4/H2 gas mixtures to lie at 7% CH4, more than twice the value found experimentally. This may suggest that the criteria governing the boundary between the diamond and non-diamond growth regions may be slightly different for mixtures containing little (or no) oxygen.
The three regions (no growth, diamond growth and non-diamond growth) of the Bachmann diagram have been explored further, via simulation of 38 different input gas mixtures using the CHEMKIN computer package and an assumed gas temperature of 2000 K. The no-growth region is shown to correspond to gas mixtures producing extremely low (<10-10) CH3 mole fractions. Diamond growth is only possible for C, H and O gas mixtures which yield both sufficiently high (~10-6) CH3 mole fractions to enable deposition of diamond and an H atom mole fraction (defined relative to that of C2H2) that is sufficient to etch the non-diamond phases. The non-diamond growth region at Tgas = 2000 K is found to span gas mixtures where [CH3] > 10-7 but [H]/[C2H2] < 0.2, under which conditions deposition is presumed to outpace the etching of non-diamond phases.
The work presented here has been published in greater detail, as referenced below:
J.R. Petherbridge, P.W. May and M.N.R. Ashfold , "Modeling of the gas-phase chemistry in C-H-O gas mixtures for diamond Chemical Vapor Deposition" J. Appl. Phys., 89 (2001) 5219-23.
![]()
 Back to Diamond CVD Home page.
Back to Diamond CVD Home page.