 Modelling of the gas-phase chemistry in C-H-O gas mixtures
Modelling of the gas-phase chemistry in C-H-O gas mixtures
Diamond growth from C-H-O gas mixtures
Low-pressure diamond deposition has been achieved using a large range of C, H and O containing gas mixtures. Bachmann et al summarized the results of many deposition experiments involving various gas mixtures and reactor types in the form of an atomic C-H-O phase diagram (Fig. 1). They concluded that the exact nature of the source gases was unimportant for most diamond chemical vapor deposition (CVD) processes and that, at typical process temperatures and pressures, it was only the relative ratios of C, H and O that controlled deposition.
The diagram partitions into three distinct regions associated with: (a) diamond growth, centred on the C-O tie line where the input mole fractions of carbon and oxygen are equal (i.e. [C] = [O]); (b) no growth, lying below the C-O tie line (i.e. [C] < [O]); and (c) non-diamond growth, located above the C-O tie line (i.e. [C] > [O]).
Previous work within our group, concerning diamond growth from CO2/CH4 gas mixtures, has confirmed the presence of three such regions. This work also involved the modeling of gas-phase chemistry for the entire range of CO2/CH4, using the CHEMKIN II computer package. Figure 1 shows a schematic of the Bachmann C-H-O atomic phase diagram with the full range of CO2/CH4 gas mixtures shown as a line cutting the diagram.
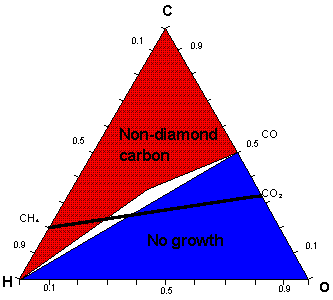
Fig. 1. Simplified atomic C-H-O diamond deposition phase diagram. The white area lying above the CO-H tie line is the experimental diamond growth domain. The thick CH4-CO2 tie line corresponds to the full range of CO2/CH4 gas mixtures
Conclusions
The previous gas-phase chemistry simulations of the CO2/CH4 system (mentioned above) have been repeated but for a large range of binary gas mixtures. All combinations of the hydrocarbons C2H2, C2H3, C2H4, C2H5, C2H6 and CH4, with each of the species, CO, CO2, H2O, OH and HO2 were considered, as well as CH4/H2 and C2H2/H2 mixtures.
Simulations were carried out using the SENKIN code which predicts the gas phase species mole fractions for a given reaction scheme, gas temperature, pressure and reaction time. No transport of reactants into or out of the reaction volume is considered. The three regions (no growth, diamond growth and non-diamond growth) of the Bachmann diagram have been explored further, via simulation of 38 different input gas mixtures using the CHEMKIN computer package and an assumed gas temperature of 2000 K. The no-growth region is shown to correspond to gas mixtures producing extremely low (10-10) CH3 mole fractions. Diamond growth is only possible for C, H and O gas mixtures which yield both sufficiently high (~10-6) CH3 mole fractions to enable deposition of diamond and an H atom mole fraction (defined relative to that of C2H2) that is sufficient to etch the non-diamond phases. The non-diamond growth region at Tgas = 2000 K is found to span gas mixtures where [CH3] > 10-7 but [H]/[C2H2] < 0.2, under which conditions deposition is presumed to outpace the etching of non-diamond phases.
For more information, see this publication.
