

|
The Chemiluminescence of Luminol - Declan Fleming - University of Bristol |
||||
|
|
Experimental Suggestions
|
Luminol Solution |
Oxidising Solution |
Reaction Characteristics |
|
- With Copper(II)
|
- With Peroxide
|
Very bright, fast reaction |
|
|
- With Cobalt(II)
|
Less bright but lasts longer - more useful experimentally
|
|
-with Bleach
|
Lasts quite long and is reasonably bright, experiment with different bleaches for the best result |
| Luminol / Base / H2O2 | Potassium Persulphate | This is too weak to be of any use experimentally |
|
-With K3[Fe(CN)6]
|
This is the reaction I worked with, I spent a week tweaking the measurements and found these to be optimum with the chemicals I used. A lasting glow of more than 50 seconds can be produced. N.B. If you are using old school supllies, check that your luminol is reasonably fresh. Luminol will go grey over time and I found this to be reasonably useless - it should appear a light olive green colour. Also, the catalyst in this reaction is strongly coloured causing the light to appear green. |
If your school has palm-top computers with scientific interfaces then
this will make your job a lot easier. For my project I was able to create
a basic light detector by using an LDR (light dependant resistor) connected
through a support system to a palm-top which could be adjusted to take
and automatically log measurements of light intensity over a set period
and with set intervals. After calibrating the LDR with a light source
of known intensity, I was able to set-up the equipment, press “go”
and after thirty seconds I had more than 250 sets of data in a spreadsheet.
It should be noted that often, an increase in rate will compromise total light yielded (light sticks can glow for weeks in a freezer).
Without this equipment a more traditional (but less accurate) approach can be adopted by laying out a long thin piece of transparent tubing around a clamp stand with a funnel at the top through which to pour your reactants. An estimation of relative rate can be made by noting the time or point on the tube at which luminescence stops.
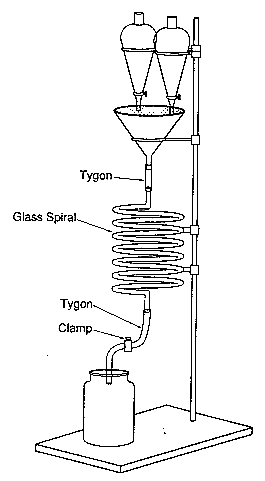
Tip: Always try to secure your glassware and spend a bit of extra time putting items necessary for the reaction in easily accessible places because you are likely to spend a lot of time fumbling around in the dark.
Concentration
We’ll start with the obvious ones, this is GCSE stuff. The more
concentrated your solution is, the more light it will produce (unless
of course there is a significant amount of colour from your reactants
– in this case there will be an awkward relationship as concentration
increases because your solution will begin to absorb its own light). This
is obviously due to the fact that there are more molecules reacting, producing
more light. One thing you might want to explore is the Beer-Lambert Law.
This should be in textbooks but will allow you to make a quantified analysis
of increasing light production.
Temperature
Temperature is another obvious one. Higher temperature means a higher
rate. For “mickey mouse” points you could show that the reaction
is less bright in an ice bath than in a warm water bath. Note that not
all chemiluminescent reactions get faster with increasing temperature
(do chemistry at university to find out about this one).
The rate of the reaction of luminol is a little more complicated than
you will have come across in school but I will attempt to explain it using
some interesting concepts.
Talking about the Arrhenius rate law will no-doubt get you points so do
this and you should be able to get a good mark. For an excellent mark,
read on.
The Steady-State Approximation
The luminol reaction could be seen as being made up of two steps; the
attack by base and then the subsequent oxidation (the last step is so
fast that it will have no effect on the overall rate). To explain the
diagram, the first step is in equilibrium so will have a forward and reverse
rate constant, k1 and k-1. The second step has rate constant, k2.
The overall rate law is going to be a combination of both these steps but because there is a lot more water around in an aqueous solution than there is oxygen, k-1 will be much larger than k2. (A) is going to be an awkward thing to measure, it will proceed to products very quickly and therefore be at low concentrations and very short lived.
The steady-state approximation allows us to deal with this problem by assuming that the concentration of (A) will be low and steady (or in other words d[(A)]/dt will be approximately zero).
Writing the rate law in terms of (A) (d[(A)]/dt) allows us to remove it by saying that this is zero.
The rate law is d[(A)]/dt = k1[luminol][NaOH]2 – k-1[(A)][H2O]2 = 0
Which becomes (remembering that water has unit activity (ideal concentration)),
k1[luminol][NaOH]2 = k-1[(A)]
(White et al have shown that the reaction is strictly pseudo first order (reaction behaves as first order although may not necessarily be first order, this might occur when one rate constant is much smaller than another and becomes negligible in comparison) where k' = 2.5 x 10-2 sec-1)
pH
I found chemiluminescence to only appear at pHs higher than 8-9 and found luminol to only dissolve easily at approximately 11.
Dyniewski et al have shown that three separate pKa values exist at pH = 1.5, 8, and 11.8 corresponding to each of the three hydrogen atoms able to dissociate.
Due to this, complete dissociation wil occur at around pH 11.8 making the luminol fully ionised and the most soluble in aqueous solvent. Below pH 8, the dissociation necessary for the reaction to proceed cannot occur and so there is no luminescence.
The pH chemistry of luminol chemiluminescence is very complicated and I failed to get any kind of conclusive data from my own experiments.
Addition / Concentration of Catalyst
Addition of catalyst will increase the rate of reaction, producing a brighter reaction that may produce less light overall. Most catalysts are based on transition metals because of the large diffuse d-electron orbitals that these compounds contain. The reactants can bond to the metal centre and interact with its different orbitals causing bond weakening within the reactants which lowers the activation energy of the reaction and increases the speed (cf Arrhenius).For a further investigation into luminol, try investigating electrochemiluminescence.