| |
Making your own Superconductors
|
|
With the advent of high temperature superconduction, it is relatively
simple to prepare and use a ceramic high temperature superconductor
in most sixth form/college science labs.
What follows are brief instructions for making an yttrium-barium-copper-oxide
superconductor - these are taken from the instructions provided
with a superconductor fabrication kit that was marketed by Colorado
Futurescience; Colorado Futurescience no longer make this kit, and
so made the instructions available on the web at http://www.webcom.com/cfsc/scpart1.html.
The method is typical of ceramic processes in scientific research.
This obviously means that these instructions are reproduced here
for purposes of interest, and I can't accept any responsibility
for their use.
For other instructions and commercial superconductor kits, check
out the 'Play' section
at Superconductors.org, which has links manufacturers and retailers.
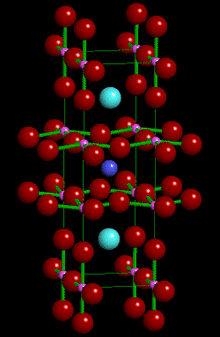
Left: Crystal structure of YBa2Cu3O7
- the so-called "1-2-3" superconductor. Click to open
a 3D VRML structure which you can rotate around.
(You will need a suitable VRML plugin
for this - the latest is SGI's Cosmo Player available at http://www.cosmosoftware.com/download)
|
Equipment
To make an yttrium-barium-copper-oxide superconductor, you will need:
Method
There are a number of methods of producing ceramic superconductors like
this, but the simplest is the so-called "shake and bake" method,
which involves a four step process:
-
Mixing the chemicals;
-
Calcination(the initial firing);
-
The intermediate firing(s) (oxygen annealings);
-
The final oxygen annealing.
The number of intermediate firings and the length of the firings are
largely up to the user. In general, the more intermediate firings, and
the longer the duration of the firings under oxygen flow, the better the
superconductor. But definite signs of superconductivity can usually be
obtained without any intermediate firing at all. In fact, if the initial
mixing of the chemicals is sufficiently thorough, the intermediate firing
is not necessary at all.
-
1. Mixing the chemicals
-
The starting mix is a grey powder made by thoroughly mixing yttrium
oxide, barium carbonate and cuprix oxide in the ratios 1:2:3 (This
superconductor is often referred to as "1-2-3" as a result)
-
-
- Yttrium Oxide, Y2O3 - 11.29 grams
- Barium Carbonate, BaCO3 - 39.47 grams
- Cupric Oxide, CuO - 23.86 grams
-
2. Calcination
- For the initial heat treatment, called calcination, the mix is heated
at 925-950 degrees Celsius for about 18-24 hours. This first treatment
may be done in a crucible or evaporating dish made of alumina or of
a good grade of laboratory porcelain. This forms the basic crystal structure
of YBa2Cu3O6.5, and gets rid of the
carbon dioxide from the barium carbonate. (Barium carbonate is used
instead of barium oxide because barium oxide of any reasonable purity
is difficult to obtain. Also, exposing barium oxide to air tends to
quickly convert much of it to barium carbonate and barium hydroxide.)
The result of this first firing is a porous black or very dark gray
clump. The coloration should be fairly even. An uneven green coloration
is an indication that the powders are not as thoroughly mixed as they
should have been, and that extra time and care should be taken to insure
thorough grinding and mixing on subsequent steps. The material will
seem to shrink rather dramatically during the initial firing as it loses
its carbon dioxide and becomes much denser than the original powder
mix.
-
3. Intermediate firing(s)
-
The porous black clump is ground into a fine powder and placed in
the furnace in an alumina dish. After the furnace temperature reaches
about 500 degrees Celsius, begin a slow flow of oxygen into the furnace.
This heat treatment under oxygen flow is called oxygen annealing.
A final furnace temperature of 925 to 975 degrees Celsius is recommended
for the intermediate firings. A temperature much higher than this
will result in a material that is much harder to re-grind. Temperatures
above 1030 degrees Celsius may destroy the crystal structure.
After the mix has heated in the furnace for at least 18 hours at
925-975 degrees Celsius, reduce the temperature slowly. If you plan
to test the sample for superconductivity after this firing, the cooling
rate must be no more than 100 degrees per hour until 400 degrees Celsius
is reached. The rate of cooling from 400 degrees down to room temperature
can be increased to about 200 degrees per hour. If you do not plan
to test for superconductivity after this firing, a cooling rate in
excess of 100 degrees per hour may be used; however a cooling rate
in excess of 250 degrees per hour is not recommended. Do not remove
the oxygen flow until the indicated furnace temperature has fallen
below 400 degrees Celsius.
The material should be thoroughly re-ground in a mortar and pestle
(or similar device) between each firing. (If, after an intermediate
firing, there is some green coloration in the resultant disk, it is
important to take extra time and care in re-grinding and mixing the
material before the next firing.) Problems that occur in the mixing
and grinding process in any of these steps are often due to hard,
coarse particles being mixed in with the finely powder material. An
ordinary kitchen tea strainer can come in handy at this point to separate
the coarser particles or lumps so they may be ground separately. IMPORTANT:
If you an ordinary tea strainer, make sure it is made of a non-magnetic
material, or make sure you are satisfied that none of the material
in the sifter or strainer will contaminate the chemicals. Even very
small quantities of magnetic materials in the chemical mix can diminish
or destroy the potential superconductivity. (It is also for this reason
that "ceramic grade" chemicals, which tend to have iron impurities,
are not often usable for making superconductors.) Shortcuts in grinding
the materials, such as using an electric coffee grinder, often contaminate
the compound with elements that destroy the superconducting properties.
Some contaminates will destroy superconductivity in very tiny amounts.
To keep your chances of success high, grinding with a good-quality
mortar and pestle is the best method. This manual grinding can be
an arduous process, but the results are worth the trouble.
-
4. The final oxygen annealing
-
The sample should be thoroughly reground, and the resultant black
powder placed back in the alumina dish. The thickness of the layer
of loose powder in the dish should match the desired thickness of
the final superconducting disk. For this final firing, the powder
should be as finely-ground and as densely-packed as possible. Do NOT
pack the powder into the dish by pressing on it from the top (as this
can makes the superconductor tend to stick to the alumina dish). Better
results can usually be obtained by tapping the alumina dish with a
pestle or a similar object so that the particles of the mix settle
together in an evenly packed disk.
For this final heat treatment, heat the sample to between 950 degrees
and 1000 degrees Celsius for about 18 hours. The higher temperature
is better, but be sure of the accuracy of your temperature indicator
before getting too close to 1000 degrees. Temperatures above 1020
degrees risk decomposition of the crystal structure and the possiblity
of the material sticking to the alumina dish. On the other hand, a
final oxygen annealing at only 950 degrees Celsius will yield a superconductor
that will crack easily, but will otherwise be satisfactory.
It is absolutely necessary that the cool-down take place very slowly
and under adequate oxygen flow. The rate of cooling must be no more
than 100 degrees Celsius per hour, especially during the critical
temperature region between 750 and 400 degrees Celsius. Take special
care to insure that the sample has access to plenty of oxygen, especially
in during the cool-down from 900 to 300 degrees. Brief interruptions
in oxygen flow when the material is above 900 are unimportant, but
continuous flow must be maintained during cool-down. If the atmosphere
in the furnace is not oxygen-rich while the sample is still above
about 400 degrees, the material can lose vital oxygen from its crystal
structure. After the furnace temperature reaches about 500 degrees,
the rate of cooling can be increased.
During this final heat treatment, a superconductor that is more
resistant to cracking during thermal stress can be produced by subjecting
the sample to high-temperature thermal cycling. To do this, vary the
temperature between 750 and 1000 degrees at rates of change of about
200 per hour. Then raise the temperature to about 1000 degrees for
an hour or more before beginning the final slow cool-down. This thermal
cycling is not a necessity at all, but it will add significantly to
the mechanical strength of the sample.
Testing the superconductor
The most foolproof test for superconductivity is the simplest. This is
the test for diamagnetism using small rare earth magnets made of samarium-cobalt
or neodymium-iron-boron, as seen in the QuickTime
video on the What Is Superconductivity?
page. Use a very small rare-earth magnet at first. Start with a rare-earth
disk magnet about 6 mm. in diameter. If you have made a good-quality superconductor,
the magnet will levitiate at least 3 mm. above the surface of the superconducting
disk. A superconductor with poor levitation can usually be improved by
re-grinding it and giving it an additional oxygen annealing.
When a superconductor levitates a magnet, a magnetic mirror image is
formed in the superconductor of the levitating magnet due to the exclusion
of the magnetic field (the Meissner
effect). The magnetic mirror image insures that there is always a
north pole induced in the superconductor directly below the north pole
of the levitating magnet. There is a south pole induced in the superconductor
directly below the south pole of the levitating magnet. This mirror image
moves with the magnet as the magnet is moves, so that the disk magnet
can be given a rapid spin without affecting its levitation. In fact the
magnet may continue to spin for quite a long time because its spinning
encounters no friction other than the friction of air resistance.
|
