| |
Superconductivity Theory - Background
To explain the properties of superconductors, it is first neccesary to
look at how normal conductors behave:
Electron Conduction Transport
Metals conduct electricity via delocalised electrons within the metal
lattice - in a metal, the atoms lose valence electrons to form a lattice
of positively-charged cations. The valence electrons are then delocalised
throughout the lattice, and are free to move between the cations - these
electrons are the current carriers.
The simplest way of explaining conductivity is by using the Drude
model. The Drude model makes the assumption the conducting electrons
-
do not interact with the cations (the "free electron
approximation"), except for collisions, where the
velocity of the electron abruptly and randomly changes direction as
a result of collision ("relaxation time approximation");
-
maintain thermal equilibrium throughout collisions ("classical
statistics approximation");
-
do not interact with each other ("independent electron
approximation").
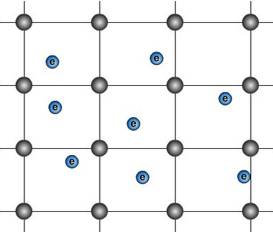 |
Left: The Drude model approximates the metal
to a lattice of cations through which delocalised electrons flow. |
For our purposes, it is necessary to adopt a modified instance of the
Drude model, whereby the electrons are assumed to have zero electrical
potential between the cations, but near the cations the potential is negative
- that is, the free electron approximation outlined above is not adopted.
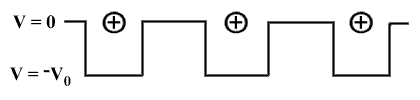 |
|
Above: Abandoning the free electron approximation: the potential
is negative near the cations and zero in the region between ions.
(Reproduced from 'Superconductivity',
Poole, Farach, Creswick, Academic Press, 1995)
|
Electron-Phonon Interaction
The cations within the lattice are oscillating about their equilibrium
positions due to thermal energy. The resulting propagating lattice vibrations
are called phonons, as they are essentially sound
waves.
The electrons then interact with the cations as they move through the
lattice - causing charge distortions that propagate along the lattice
structure, in turn causing distortions in the periodic potential.
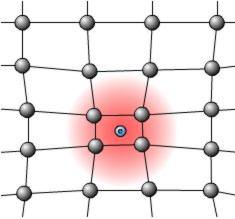 |
Left: Lattice distortion around
an electron causes an increase in positive charge density that will
propagate along the lattice with the cation vibrations. |
These distortions can affect the motion of another electron at some distance
that is also interacting with the lattice in a similar way - this is thus
called an electron-phonon interaction, and is
an integral part of Cooper pair
formation.
From this, it is simple to see why conductivity decreases with temperature
- increased thermal energy will cause the cations to oscillate more violently;
the electron-phonon interactions are greater and so impede the flow of
electrons through the lattice. Conceptually, it is simplest to visualise
this as the cations physically 'knocking' the electrons off their paths:
|