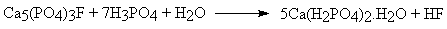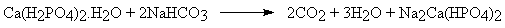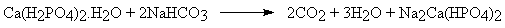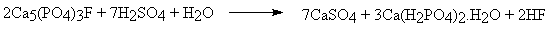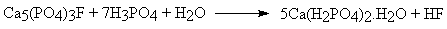THE ACTION OF BAKING POWDER
- 'Straight baking powder' is a mixture of monocalcium phosphate monohydrate and sodium hydrogen carbonate:
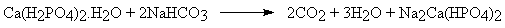
- However this tends to produce carbon dioxide too quickly during dough mixing and so 'combination baking powder' is preferred:
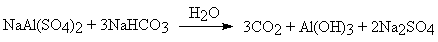
PRODUCTION OF "SUPERPHOSPHATE"
- "Superphosphate" is now made by the highly expthermic addition of sulphuric acid to fine-ground phosphate rock:
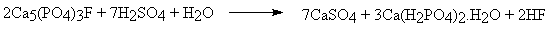
- Here the calcium sulphate produced is an unwanted byproduct, which can be avoided by using phosphoric acid instead of sulphuric acid, producing "triple superphosphate":