![]()

Heisenberg
Uncertainty Principle
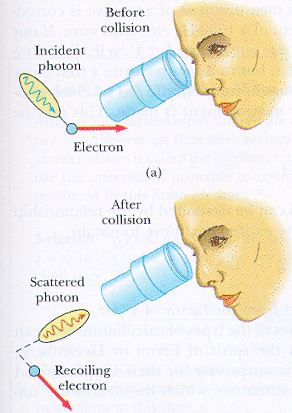
Have you ever heard of the Heisenberg uncertainty principle? I was told about it when I was studying A-Level Chemistry, it basically states that the more you know about the momentum of a particle the less you know about the position and vice versa.
Why should that be? Doesn't that strike you as strange? The very act of determining a particle's position means that you cannot know it's velocity.
|
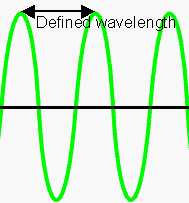
Another way of looking at this is by considering wavefunctions. If you know the exact wavelength of a particle, then you know it's momentum. However a particle with a known momentum has a totally unpredictable location. You can see this if you look at the graph left - the electron is equally likely to be anywhere along the wave. |
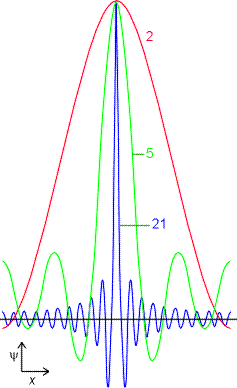
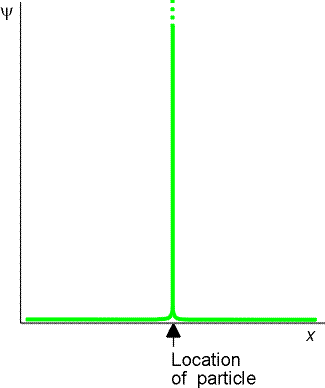
The wave function of a perfectly localised particle would be a sharply spiked function at the particles position and zero everywhere else. This can be aproximated by the addition of of several waves, which interfere constructively in one place and destructively in all others. However in doing this you loose information about the momentum of the particle - there is no well defined wavelength.
 |
 |
As more waves are used in the supposition (given by numbers on curves) the location becomes more precise at the expense of uncertainty in the particles momentum |
An infinite number of waves is needed to construct the wavefunction of a perfectly localised particle - so there is total uncertainty in the momentum |
Important things to note:
Due tothe small value of h in everyday units, this principle is only significant on the atomic scale.
The uncertainties of Dx and Dp arise from the quantum structure of matter, and are not due to imperfections in the measurement instruments.
graphs taken from: Physical Chemistry, Peter Atkins, 6th Ed, OUP (1998)
This page has been created by Peter White, June 2001