School
of
Chemistry
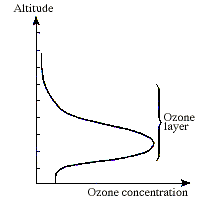
Earth's AtmosphereAfter the formation of Earth, approximately 5 billion years ago, a dense atmosphere emerged during the degassing of the planet's interior. This atmosphere probably consited of hydrogen, methane, water vapour and carbon oxides. The most important feature of this environment was the absence of free oxygen. The evidence for this oxygen-free atmosphere can be found in early rock formations which contain many elements, such as iron and uranium, in their reduced states. The elements are not found in this state in rocks less than 3 billion years old. Condensation of the water vapour about 4 billion years ago resulted in the formation of the oceans. Approximately one billion years ago aquatic organisms began to use sunlight to convert molecules of H2O and CO2 into organic compounds and molecular oxygen. In the atmosphere some of this molecular oxygen absorbed ultraviolet radiation from the sun, which caused dissociation into single oxygen atoms. The monatomic oxygen then reacted with remaining oxygen molecules to form ozone. By about 600 million years ago the amount of ozone in the atmosphere was sufficient to shield Earth from biologically lethal UV radiation. This allowed the evolution of organisms which could live on land. The present day atmosphere consists mainly of nitrogen (78%), oxygen (21%) and argon (<1%). The atmosphere is divided into different layers, depending on the temperature gradient. The troposphere is the layer from the surface up to about 11km, in which the temperature falls with increasing altitude. The stratosphere is the layer above this, up to about 50km, where the temperature gradient is reversed. The ozone concentration peaks in the stratosphere at an altitude of 20 - 30km. This is known as the ozone layer.
The Ozone layer (image taken from www.nobel.se/chemistry/laureates/1995/press.html) |

 |
 |
 |
 |
 |
 |