|
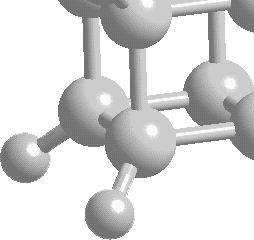
It was mentioned
previously that cubane was first prepared in 1964 by Dr. Philip
E. Eaton. He was partnered by Thomas W. Cole and together
they successfully completed the first synthesis, shown schematically
below:
Hint:
Run the cursor over the image for further details of the synthesis.
image of molecule taken from
http://cst-www.nrl.navy.mil/lattice/struk/c8h8.html
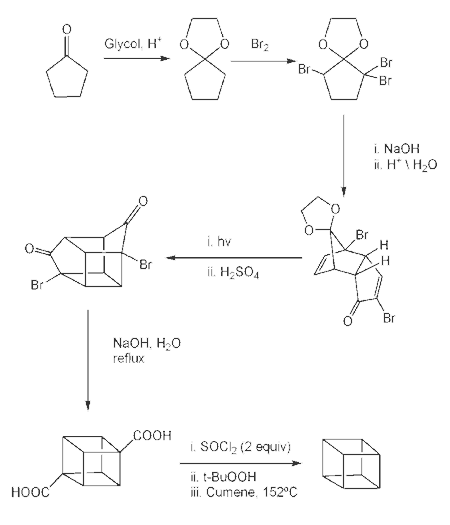
This,
however, was soon simplified by N.B.Chapman who condensed
the process to give cubane-1,4-dicarboxylic acid in five steps
and so cubane in six:
In
1966 J C Barborak et al discovered yet another new
synthesis of cubane. It was slightly unconventional in the
fact that it utilised cyclobutadiene as a key substance to
the process. Before this,cyclobutadiene was usually unavailable
for the purposes of organic chemistry due to it's instability.
The shorter synthesis is shown below:
Since
the synthesis of the cubane-1,4-dicarboxylic acid has become
shorter and easier, a new decarboxylation method has also
devised to give increased yields of the final cubane product.
This has allowed the scale of production reach multikilogram
batches in places (Fluorochem in California and EniChem Synthesis
in Milan) eventhough cubane and its derivatives remain expensive
to purchase.
|