

--------------------------
Antimony Facts
--------------------------
| Physical Properties | Chemical and Crystal Properties | |||
|---|---|---|---|---|
 |
 |
|||
| Colour | Tin-white to steel-grey | Atomic number | 51 | |
| Lustre | Metallic | Symbol | Sb | |
| Melting point/ºC | 630.63 | Atomic weight (12C)/gmol-1 | 121.757 | |
| Boiling point/ºC | 1587 | Electronic configuration | [Kr]4d105s25p3 | |
| Thermal conductivity/Wcm-1K-1 | 0.243 | Atomic radius/Å | 1.53 | |
| Natural abundancy | 0.2-0.5ppm | Covalent radius/Å | 1.38 | |
| Hardness (Mohs) | 3-3.5 | Electronegativity (Pauling) | 2.05 | |
| Hardness (Brinell)/MNm-2 | 294 | Crystal system | trigonal | |
| Electrical conductivity/×106cm.Ohm | 0.0288 | Crystal habits | pseudocubic rhombohedral | |
| Density/gcm-3 | 6.684 | Occurrences | see here | |
| Heat of fusion/kJmol-1 | 19.87 | Minerals | see here | |
| Vapour pressure/10-9Pa @ 6304K | 2.49 | Nature of oxide solution | mildly acidic | |
| Heat of vaporisation/kJmol-1 | 77.14 | Common oxidation states | 5, 3, -3 | |
| Molar volume/10-6m3mol-1 | 18.19 | Known isotopes | 121Sb (stable - 70 neutrons) 57.36% abundant | |
| 1st ionization enthalpy/kJmol-1 | 834 | 123Sb (stable - 72 neutrons) 42.64% abundant | ||
| 2nd ionization enthalpy/kJmol-1 | 1595 | 125Sb (synthetic, unstable*) | ||
| 3rd ionization enthalpy/kJmol-1 | 2440 | 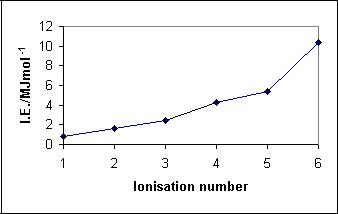 |
||
| 4th ionization enthalpy/kJmol-1 | 4260 | |||
| 5th ionization enthalpy/kJmol-1 | 5400 | |||
| 6th ionization enthalpy/kJmol-1 | 10400 | |||
* decays via beta radiation with a half-life of 2.7582 years to 135Te.