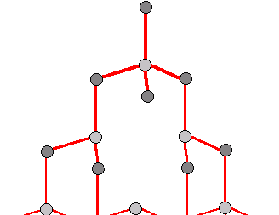
 Figure 2a: The structure contains a hole where an atom should be creating a
vacancy
Figure 2a: The structure contains a hole where an atom should be creating a
vacancyCrystal Defects and Impurities
There are three main crystal defects shown in figures 2a, 2b and 2c.
 Figure 2a: The structure contains a hole where an atom should be creating a
vacancy
Figure 2a: The structure contains a hole where an atom should be creating a
vacancy
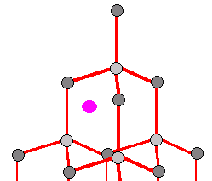 Figure 2b: An atom is present in a space not meant for it. These atoms are
known as "interstitial" atoms.
Figure 2b: An atom is present in a space not meant for it. These atoms are
known as "interstitial" atoms.
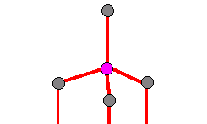 Figure 2c: The
structure contains impurities - the wrong atom is substituted for a carbon atom.
Examples include Cr
and Mg which can be substituted for similar sized atoms.
Figure 2c: The
structure contains impurities - the wrong atom is substituted for a carbon atom.
Examples include Cr
and Mg which can be substituted for similar sized atoms.
Single atoms of nitrogen can be substituted for carbon atoms and as nitrogen has one more electron than carbon, this “extra” electron has to go somewhere. All of carbons electrons are already in covalent bonds so these “extra” electrons remain unpaired.
A carbon atom has the electron configuration 1s2 2s2 2p2 so there are 4 electrons in its outer shell. This leaves empty p-orbitals to which electrons could theoretically move to, but only if the diamond crystal was given enough energy. Under normal conditions, this does not happen because the amount of energy needed to excite the electrons is too large. However, the presence of the “extra” electrons from nitrogen decreases the amount of energy needed, and this is approximately equal to that of light in the blue/violet part of the spectrum.
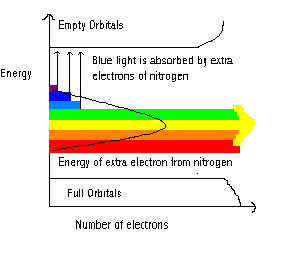 Figure 3: Colour of a diamond structure containing nitrogen
Chemistry Review Magazine Volume 10,
Number 4, April 2001
Figure 3: Colour of a diamond structure containing nitrogen
Chemistry Review Magazine Volume 10,
Number 4, April 2001
Therefore if you shone blue/violet light onto a crystal structure containing isolated nitrogen atoms, the “extra” electrons provided by nitrogen would absorb this energy and be excited to the empty orbitals. If white light, which contains all wavelengths in the spectrum, is shone onto the crystal, the electrons absorb the blue/violet light but the remaining wavelengths pass straight through the structure. It is this light that we observe so the crystal appears to be yellow. These intense yellow diamonds are quite rare and are called canary yellow.