 The famous Tiffany Diamond
http://www.original-diamonds.com/famous_tiffany.php
The famous Tiffany Diamond
http://www.original-diamonds.com/famous_tiffany.phpDiamonds
 The famous Tiffany Diamond
http://www.original-diamonds.com/famous_tiffany.php
The famous Tiffany Diamond
http://www.original-diamonds.com/famous_tiffany.php
Diamond has a basic structure with each carbon atom joined to four others by covalent bonding, forming a tetrahedron.
This structure explains some of the physical properties of diamond, for example its hardness and its high conductivity of heat. However, it does not explain the fact that diamond exists in many different colours. In order to determine the reason for this, the structure needs to be examined more closely. A perfect diamond structure would have one as shown in figure 1.
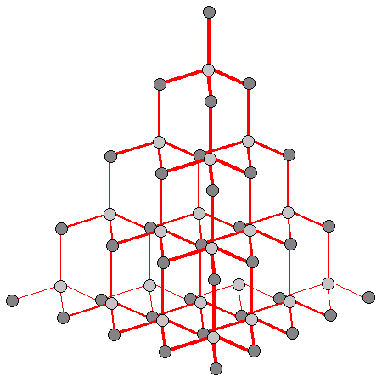 Figure 1: The Structure of Diamond
Figure 1: The Structure of Diamond
http://www.uwgb.edu/dutchs/PETROLGY/Diamond%20Structure.HTM
In reality all crystals contain anomalies in their structures known as crystal defects.
Diamonds are the jewellery of royalty and it is not surprising when you look at the brilliant colours of these sparkling gemstones.