![]()
White Phosphorus
The fiery element discovered by an alchemist.
![]()
Roderick Edmonds
Eton College, UK
![]()
Molecule of the Month July 2023
![]()
|
White PhosphorusThe fiery element discovered by an alchemist.
Roderick Edmonds
Molecule of the Month July 2023
|
 |
I thought phosphorous was an element not a molecule?First, the spelling is phosphorus, not phosphorous - a common mistake. And phosphorus is an element that exists as P4 molecules. Ok, fair enough. But weren’t alchemists trying to make gold?Yes – and Hennig Brand, in 1669, thought a promising starting material would be a gold-coloured liquid excreted by the human body and so, perhaps, imbued with some vital or magical energy. His recipe involved leaving a pot of urine to putrefy for a week or two, then heating it in several stages to progressively higher temperatures. Carbon compounds in the urine were oxidised to carbon monoxide, reducing the phosphates present to white phosphorus. This discovery inspired the famous painting shown, but this impressive work of visual art cannot convey the unsavoury nature of the process. It is said that his investigations were funded by his wealthy wife, whose spousal devotion to these malodorous endeavours one cannot but admire. |
 The Alchemist Discovering Phosphorus; Joseph Wright of Derby, 1771. |
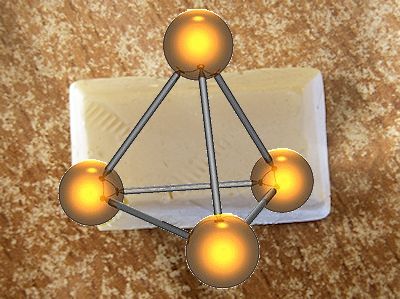
It’s a soft solid, composed of tetrahedral molecules of formula P4. When pure, it is white, although it usually looks yellow because the atoms gradually rearrange to form red phosphorus, a more stable allotrope of the element which has a polymeric structure. There are also various other allotropes of phosphorus, such as violet, black and blue.
 |
 |
| White phosphorus stored under water. | White phosphorus, removed from the water, glows faintly in the dark. This property gave phosphorus its name, which comes from the Greek meaning 'Light-bearer'. [Photo by kind permission of Janet Steadman] |
White phosphorus is notoriously flammable, igniting readily in air to form phosphorus(V) oxide. That’s why the small sample in the photo above is under water in a jar. If it is carefully removed, in a dark room, it can be seen to glow as the reaction of its surface with oxygen releases energy in the form of light. It will catch fire if it becomes too warm.
Strictly speaking, no – it’s chemiluminescent. Things that glow in the dark normally do so because electrons in “excited” energy levels emit light as they relax down to lower-energy states, the lowest energy of which is called the ground state. There are three common ways for this to occur:
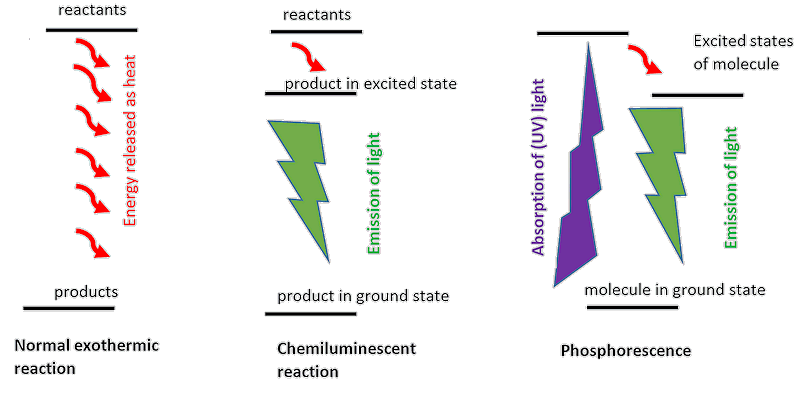
I’ve some toys that glow in the darkThey will contain a phosphorescent material, such as strontium aluminate doped with europium. After some time in the dark, the glow will fade, and you’ll need to expose them to light again to re-excite the phosphorescent substance. The glowing background to this Hallowe’en bat sticker contains, fortunately, no phosphorus. |
 |
 |
In Sir Arthur Conan Doyle’s Sherlock Holmes mystery, The Hound of the Baskervilles, the villain applies white phosphorus to a fierce dog, making it appear more terrifying when his victims encounter it in the dark. |
Yes – lots of it, and in fact the chemistry involved is similar to that in Hennig Brand’s method, although with improved starting materials. Coke is used as a source of carbon, which reduces calcium phosphate at high temperatures. The phosphorus is transported in containers like the one shown in the photo. Since white phosphorus melts at 44°C, gentle heating allows it to be pumped into or out of the container as a liquid.
 |
 |
| Rail container filed with yellow phosphorus (slightly oxidisd white P) under water. | A Coke bottle showing phosphoric acid among its ingredients. |
Most of the white phosphorus is burnt to produce phosphorus(V) oxide, which when added to water, makes phosphoric(V) acid for use in fizzy drinks. Coca Cola and Pepsi have pH values around 2.6 - the same as lemon juice or vinegar! - due to their phosphoric acid content.
Isn't phosphorus used on matches?Originally, yes, as you might recall from the British soldiers of the First World War who cheered themselves up with the rousing chorus of Pack up your troubles in your old kit-bag. The words are: "While you’ve a Lucifer to light your fag, smile, boys, that’s the style." A 'Lucifer' was an early type of match containing white phosphorus together with potassium chlorate(V) as an oxidising agent. The Lucifer matches had two drawbacks. One was their tendency to self-ignite in the pocket or backpack, before the smoker had even considered indulging in a cigarette. The other was the unhealthy conditions in the factories where they were made. |
 A box of 'Lucifer' matches [Lucifer label by kind permission of David Brewis] |
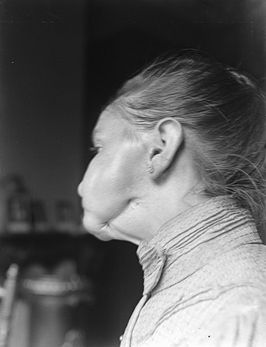 Woman with 'phossy jaw'. [From the Dutch National Archive via Wikimedia Commons.] |
How so?Small, non-polar molecules such as P4 have only very weak forces between them, so solid white phosphorus easily sublimes to a gas. Prolonged inhalation of this led to phossy jaw, a painful and debilitating condition causing tooth loss, oral abscesses, and the gradual destruction of the lower jawbone. The mechanism by which this occurs is still not clear, although it is believed that the white phosphorus reacts in the body to form compounds which interfere with the natural balance between the deposition and reabsorption of bone. Most of the match factory workers were women and girls, who were barred from many other jobs at the time. A landmark in the field of industrial health and safety came in 1888 when they staged a strike to protest against their working conditions. Fortunately, other types of match were already being developed. Modern matches contain no white phosphorus, although the less hazardous red phosphorus may be found in the striker on the side of the box. |
 Shouldn’t phosphorus be inert, like nitrogen?
Shouldn’t phosphorus be inert, like nitrogen?You may well think so, as phosphorus lies directly below nitrogen in Group 5 of the Periodic Table. School chemistry courses usually focus on the alkali metals and the halogens, rather than on those Groups in the middle where the trends are less obvious. In fact there are some similarities; nitrogen will react with oxygen, producing harmful nitrogen oxides in vehicle exhausts (see MOTM December 2012). However, the reaction is endothermic and only occurs at very high temperatures. The key reasons for the greater reactivity of phosphorus are:
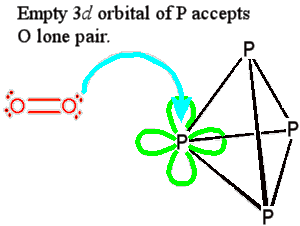 Phosphorus, unlike nitrogen, has empty d-orbitals in its valence shell which can accept an electron pair from an attacking molecule. The reaction can thus start by making, rather than breaking, a bond, and so will have a lower activation energy. Other examples of this type can be found when comparing elements from the second and third Rows; one is the easier hydrolysis of SiCl4 than CCl4 which is discussed in detail on the Chemguide Group 4 web pages.
Phosphorus, unlike nitrogen, has empty d-orbitals in its valence shell which can accept an electron pair from an attacking molecule. The reaction can thus start by making, rather than breaking, a bond, and so will have a lower activation energy. Other examples of this type can be found when comparing elements from the second and third Rows; one is the easier hydrolysis of SiCl4 than CCl4 which is discussed in detail on the Chemguide Group 4 web pages.Incendiary weapons are those designed to set fire to a target, and they are now more or less forbidden by United Nations conventions (see MOTM for December 2022 about napalm). White phosphorus is very effective as an incendiary, and unfortunately it has been put to this use in several recent conflicts. It also produces a thick cloud of phosphorus(V) oxide which makes a very good smokescreen, and concealing your activities from the enemy in this way is considered legitimate in warfare. This accepted application of white phosphorus on the battlefield makes it difficult to prosecute its illegal, incendiary, use.

A US Air Force plane drops a white phosphorus bomb in South Vietnam in 1966
[US Air Force, public domain via Wikimedia Commons]
So it’s one of the many chemicals which can be put to useful, or destructive, purposes. Another is nitroglycerine (MOTM October 2007), the explosive material in dynamite but also one of the best medicines against angina.
That is true of phosphorus compounds, particularly phosphates, rather than the element itself. Calcium phosphate is a key component of bone, and the phosphate group also forms part of the molecules of DNA (MOTM Jan 2000) and ATP (MOTM January 1998). Phosphorus compounds are ubiquitous in nature, and are recycled through plants, animals and inorganic minerals in the Phosphorus Cycle.
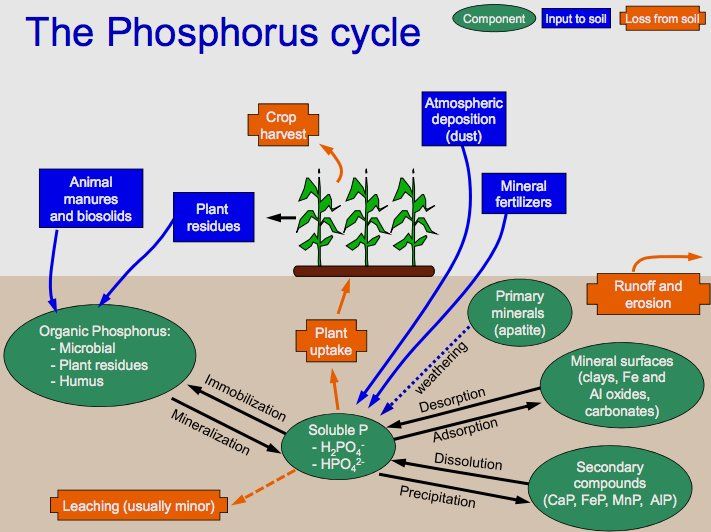
The phosphorus cycle in nature.
[Image: Welcome1To1The1Jungle via Wikimedia Commons]
Phosphates are often included in agricultural fertilisers, and Aldous Huxley’s dystopian 1932 novel Brave New World suggests that the dead would most suitably be disposed of by sending the bodies to a phosphorus recycling centre!
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.23394422]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.23394422]