Copper is an essential element in living systems, forming a large number of metalloproteins. Amoung the functions of the copper proteins are: electron transfer with either an outersphere mechanism, or functioning as an inner-sphere reductase, both involving the Cu(I)/Cu(II) couple; mono- terminal-oxidases, which form either water or hydrogen peroxide from dioxygen; oxygenases, which incorporate an oxygen atom into a substrate; superoxide degradation to form dioxygen and peroxide; and oxygen transport. The blue copper proteins have a beautiful blue colour, far more intense than that of the Cu(II) ion in water, but similar in hue. Caeruloplasmin has several of these functions, and is a blue colour, hence the recently acquired description as a "moonlighting' protein.
From a structural and spectroscopic point of view, the three main types of biologically active copper centres found in the copper proteins may be distinguished according to a generally accepted convention deriving mainly from from their electron paramagnetic resonance (EPR) spectra.
Type 1, (T1), have 'blue' copper centers, with the copper normally coordinated to two nitrogens and two sulphurs
Type 2, (T2), have 'non-blue' copper centers, with the copper coordinated to two or three nitrogens and oxygens
Type 3, (T3), have copper dimers
The nitrogens come from histidine groups, the sulphurs from methionine and cysteine, the oxygens from a carboxyllic acid in the protein. Water, hydroxide and alkoxide oxygens are also used.
The Protein Data Bank
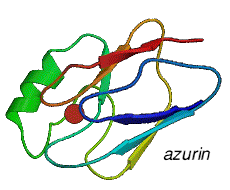
The Protein Data Bank is "the single worldwide repository for the processing and distribution of 3-D biological macromolecular structure data". The database lists a large number (280 in July 2003) of copper containing proteins. You can find these with a search. Caeruloplasmin has the PDB reference 1KCW. The picture to the right is of plastocyanin, (extracted from spinach), from a single crystal structure at a resolution of 1.7Å, (Ref: Y.Xue, M.Okvist and S.Young, April 1997, PDB number 1AG6)
Note the position of the single copper atom at the top of the structure