|
 |
 |
|
|
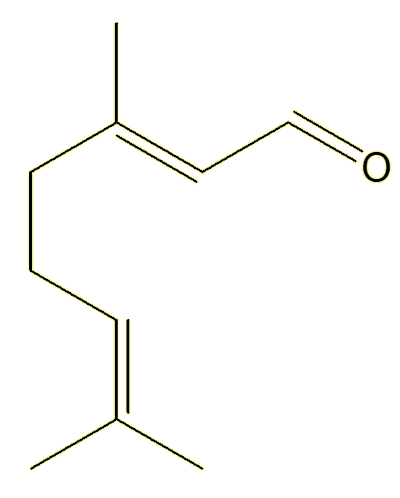
Geranial |
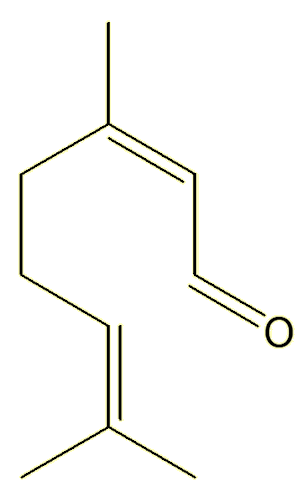
Neral |
|
I don't see a picture of a molecule of citral?
You won’t, it doesn't exist.
What does that mean?
A bottle labelled 'citral' actually contains a mixture of geranial and neral molecules.
They look similar?
Yes, they both have the same molecular formula, C10H16O, they just differ in the orientation around one of the double bonds.
Meaning?
Unlike single bonds, there is no free rotation about double bonds. The structures are ‘locked’, they do not interconvert unless you heat them to very high temperatures, so these two compounds live separate existences – and can be isolated separately. They are known as geometric isomers.
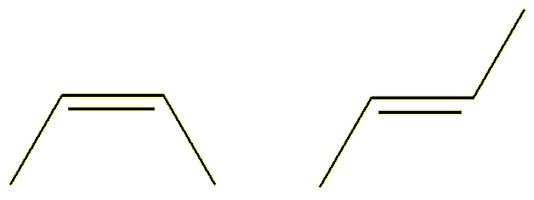 |
| (Z-) |
(E-) |
The Z- isomer (from the German zusammen, together) has the ‘backbone’ carbon chain on the same side of the double bond; the E- isomer (from the German entegegen, opposite) has the two parts of the backbone on opposite sides of the molecule. The Z-isomers is also commonly called the cis-isomer (cis = same), whereas the E-isomer is called the trans-isomer (trans = across).
 The name citral makes me think of citrus fruits.
The name citral makes me think of citrus fruits.
You would be right. It is present in many plant oils, like lemon-peel oil and lemongrass oil. Lemongrass oil is the best source of citral, often making up 70% or more of it. Lemon oil itself is mainly limonene (MOTM March 2008) but the small amount of citral is largely responsible for its smell. Similarly, it is only present as a trace in orange oil.
It is interesting that orange is a very popular flavouring, whereas lemon is more popular for its fresh smell, as in cleaning products ("lemon = 'clean'").
Do neral and geranial smell the same?
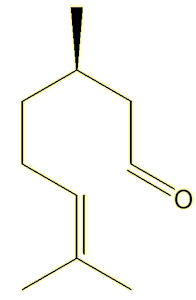
Very similar but not identical. They both have lemony, sharp, smells, but geranial smells rather fresher. Citronellal, from oil of citronella, which is used in some insect repellents (see MOTM for Jan 2011), has a rather similar smell (notice the similarity in structure to neral).
 |
|
| Citronella |
|

Green-veined white butterfly (Pieris napi)
[Photo: Charles J Sharp / CC BY-SA] |
Are these compounds important in Nature?
Quite a few insects use them in pheromones. Neral is an alarm pheromone in many types of mite. The female of the parasitic wasp Itoplectis conquisitor uses citral as a sex pheromone, whilst the male Green-veined White butterfly, Pieris napi, emits citral from wing glands, as a requisite for females to accept mating with a courting male. Both citral isomers need to be present for it to be effective. Possibly the most important, though, is Nasonov pheromone.
|
 What's that?
What's that?
It is named after the Russian discoverer, Nikolai Viktorovich Nasonov (1855-1939), pictured right, who discovered it in 1883.
It is released by (male) honeybee drones from a gland in their abdomen to recruit other males and guide swarms to new nests, for example. When drones do this, they raise their abdomens and fan their wings strongly. Citral is one component of it, a minor one which seems to be essential. Beekeepers use a synthetic Nasonov pheromone to lure swarms; citral is also essential to this.
And these molecules are important?
Yes, apart from their use in Nature, the two lemon odorants in citral, geranial and neral, have odours that are much in demand in industry. However, both these molecules are readily oxidised and more stable alternatives have been sought out for many years.
Why are these molecules so reactive?
There are three very reactive groups, a carbonyl (aldehyde) and two C=C double bonds, in each citral. Aldehydes are easily oxidised to carboxylic acids. They also react with the other specialised laboratory reagents, so in the laboratory the C=O bond can be reduced with sodium borohydride. The carbonyl group undegoes condensation reactions with chemicals like hydroxylamine. And strong oxidation with e.g. alkaline permanganate, splits up citral at the C=C bonds into three fragments: propanone (acetone); ethane-1,2-dioic (oxalic) acid; and 4-ketopentanoic acid - which was used by late Victorian chemists as a proof of the structure of a citral molecule.
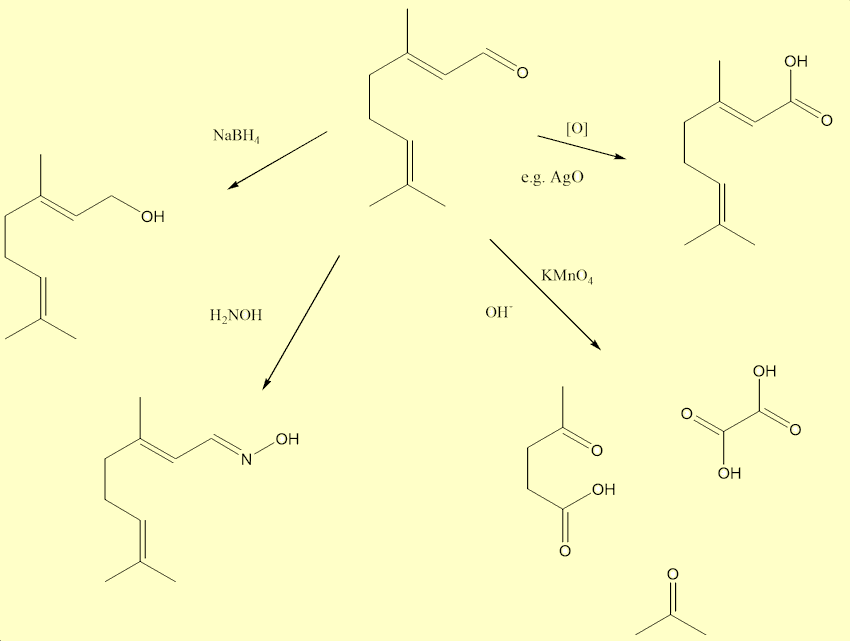
Are there any other molecules that smell like this?
Chemists have obviously looked for alternative fragrances, starting with citral-like structures. One such, which is more stable and also has a lemon smell, is geranylnitrile.
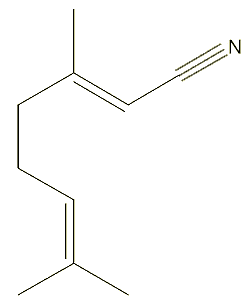 |
|
| Geranylnitrile |
|
A study of the molecules present in, of all things, fried chicken thighs, identified a range of alkyl thiophenecarbaldehyde odorants, leading to the synthesis of 35 molecules, two of which, 3-butyl-2-thiophenecarbaldehyde and 3-(3-methylbut-2-en-1-yl)-2-thiophenecarbaldehyde, had strong citrus and citral-like smells.
|
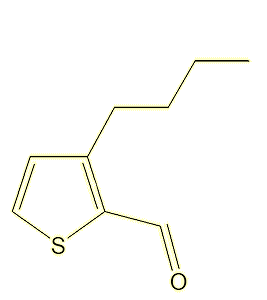 |
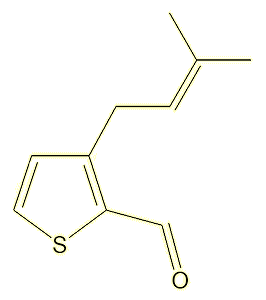 |
|
|
3-butyl-2-thiophenecarbaldehyde |
3-(3-methylbut-2-en-1-yl)-2-
thiophenecarbaldehyde |
|
How do you make citral?
Most people don't make citral in the laboratory, it is much cheaper to get it from natural sources. Over a hundred years ago, when its stricture was not known, French chemists (Barbier, Bouveault and Tiemann) attempted its synthesis, successfully, in five stages.
The first step started from 2,4-dibromo-2-methylbutane, reacting it with pentane-2,4-dione in the presence of base, which deprotonates the pentanedione at the carbon between the two carbonyls (the most acidic hydrogen). This generates a nucleophile which attacks the 2,4-dibromo-2-methylbutane forming a bromodiketone. The second step reacts this with alkali (NaOH). This makes the bromodiketone eliminate a HBr molecule, generating a desired double bond, and also causes a reverse aldol reaction of the diketone, forming 2-methylhept-2-en-6-one. The third step involved a Reformatsky reaction with ethyl iodoacetate, forming the ester ethyl geranate. The fourth step hydrolysed the ester, isolating the calcium salt of geranic acid. Finally, the fifth step was the pyrolysis of a mixture of the calcium geranate with calcium formate, which did indeed give citral - identical to material obtained from natural sources.
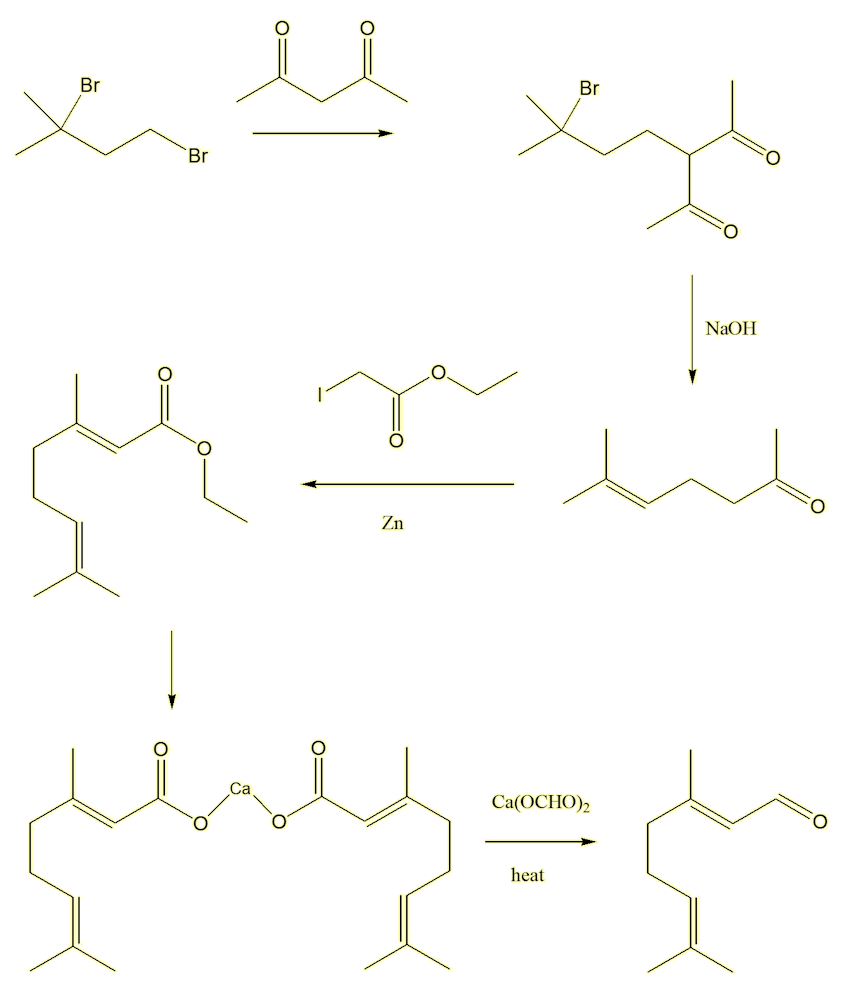
No wonder no-one makes it!
Not so! It is an important industrial chemical, used in the manufacture of Vitamin A (MOTM January 2017). A synthesis devised by the German chemical giant BASF and used in their plant at Ludwigshafen shows modern industrial synthesis at its best. It uses isobutene and methanal as starting materials, both of which are obtained from petrochemicals. The only by-product is water. The first step reacts isobutene with methanal to form the unsaturated alcohol, isoprenol. This can be isomerised into prenol or oxidised over a silver catalyst to prenal. On heating these two compounds together, they form citral in one step!
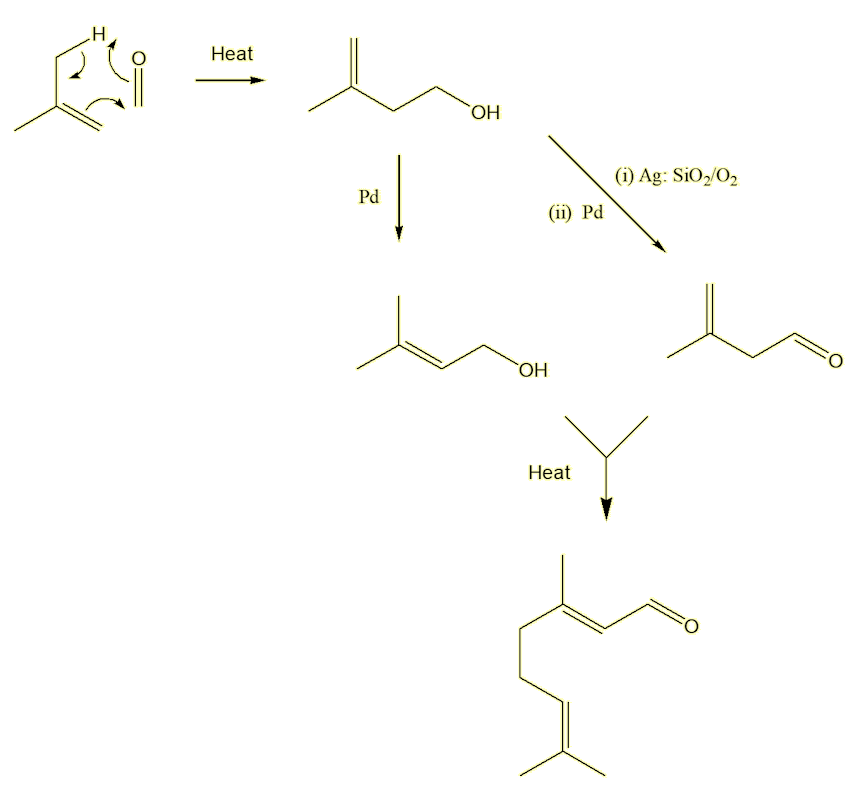

Bibliography
- Chapman and Hall Combined Chemical Dictionary, CRC Numbers: Citral JGH37-H; Geranial JFJ79-Y.
Citral - properties and synthesis
- C. S. Sell, A Fragrant Introduction to Terpenoid Chemistry, Cambridge, RSC, 2003, esp./ pp 49-54, 289-300.
- C. S. Sell, Chemistry and the Sense of Smell, Hoboken, NJ, Wiley, 2014, esp. pp. 258-259, 303-307.
Lemons
- R. M. Ikeda, L. A. Rolle, S. H. Vannier and W. L. Stanley, J. Agric. Food Chem., 1962, 10, 98-102 (aldehydes in cold-pressed lemon oil)
- P. A. Vatakencherry, K. N. Pushpakumari and J. Varghese, Perfumer & Flavorist, 1987, 12 (Oct/Nov), 23-26 (separation of citral from lemongrass oil)
- E. H. Chisowa, D. R. Hall and D. I. Farman, Flavour & Fragrance J., 1998, 13, 29-30. (composition of lemongrass oil)
- D. Reeve, Perfumer & Flavorist, May 2005, 30, 32-35 (the lemon and lemon oil)
Alternative lemon odorants
- R. J. Cannon, N. L. Curto, C. M. Esposito, R. K. Payne, A. J. Janczuk, D. O. Agyemang, T. Cai, X-Q. Tang and M. Z. Chen, J. Agric. Food Chem. 2017, 65, 5690−5699 (molecules from fried chicken)
Citral in insect pheromones
- D.C. Robacker and L. B. Hendry, J. Chem. Ecol., 1977, 3, 563–577 (Itoplectis conquisitor)
- Y. Kuwahara, 'Chemical ecology of astigmatid mites', in: Advances in Insect Chemical Ecology, R. T. Cardé and J. G. Millar (eds), Cambridge, Cambridge University Press, 2004 pp. 76–109 (neral in mite alarm pheromones)
- H. Larsdotter-Mellström, K. Eriksson, I. Liblikas, C. Wiklund, A. K.Borg-Karlson, S. Nylin, N. Janz and M. A. Carlsson, Front. Physiol., 2016, 7, 68 (Pieris napi)
Nasonov phermonone
- J. B. Free, Pheromones of Social Bees, Ithaca, Cornstock, 1987.
- W. C. Agosta, Chemical Communication, New York, Scientific American Library, 1992, pp 88-91.
- J. O. Schmidt, J. Chem. Ecol., 1999, 25, 2051-2056.
- K. N. Slessor, M. L. Winston and Y. Le Conte, J. Chem. Ecol., 2005, 31, 2731- 2745 (review)
- M. Trhlin and J. Rajchard, Veterinarni Medicina, 2011, 56, 265-273 (review)


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12362825]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12362825]
![]()
![]()
![]()
![]()




 The name citral makes me think of citrus fruits.
The name citral makes me think of citrus fruits.

 What's that?
What's that?




