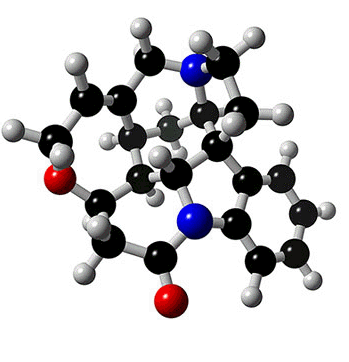
STRYCHNINE
The Performance Enhancing Deadly Poison
Paul M. Burnham
Greenhead College, Huddersfield, UK
Molecule of the Month October 2009
Also available: HTML version.
 |
STRYCHNINEThe Performance Enhancing Deadly PoisonPaul M. Burnham Molecule of the Month October 2009 |
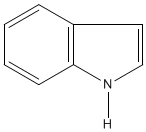
The alkaloid strychnine is a very bitter tasting white crystalline solid and a deadly poison. Alkaloids are naturally occurring compounds that contain nitrogen. The name alkaloid is derived from the basicity of the compounds and they dissolve in, and form salts from, acids. There are hundreds of alkaloids and examples include atropine, epibatidine, morphine, nicotine and quinine. Alkaloids can be classified by the chemical that they are derived from and strychnine is an example of an indole alkaloid. The structures of both indole and strychnine are shown below with the indole section of strychnine coloured red. |
 |
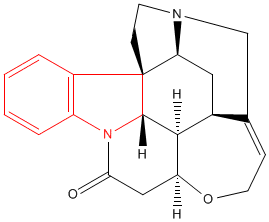 |
| Indole | Strychnine |
Due to its toxic effects strychnine is used as a form of pest control and has been employed as a rodenticide and to kill birds. However, the use of it as a form of mole control was banned from September 2006 by the EU as a result of the painful and violent death that it causes. |
Strychnine is found in the bark and seeds of the Strychnine tree or poison nut tree, Strychnos nux-vomica L., from which the compound gets its name. It is also found in the Ignatius bean, a plant closely related to the Strychnine tree, and it was from this source that the compound was first isolated in 1818 by two French chemists. Pierre-Joseph Pelletier and Joseph-Bienaimé Caventou extracted the beans with ether and, after evaporating the solvent, obtained a thick green oil. This was dissolved in hot alcohol to remove impurities by filtration. The alcohol was evaporated, the resulting residue treated with an alkali and a precipitate of strychnine was obtained. |
 |
 |
Strychnine was only the second alkaloid to be extracted, the first was morphine. Pelletier and Caventou wanted to name their new alkaloid vauqueline after Nicolas Vauquelin, one of their associates who had refined the technique of ether extraction for use in isolating alkaloids. However, the officers of the Académe des Sciences in Paris rejected the idea on the grounds that a respected scientist’s name should not be paired with a deadly poison. In addition to strychnine the pair isolated other important compounds from plants including caffeine, chlorophyll and the anti-malaria drug quinine. This made them famous and in 1970 both scientists appeared on a French stamp commemorating the 150th anniversary of their discovery of quinine. Joseph-Bienaimé Caventou has also been honoured in space, the Caventou crater on the moon is named after him. |
Despite its isolation in 1818 the exact composition and structure of strychnine was not known for many years, in part due to its complex structure of seven rings and six asymmetric centres. Eventually in 1947 after many experiments and hundreds of publications the precise structure was reported. The next task of the chemists was to design an effective method to synthesise strychnine from readily available materials. This was achieved by Robert Woodward and his research group who communicated their route in 1954 and published a full report in 1963. They synthesised strychnine from 2-veratrylindole in a route that involved twenty six separate reactions. Many other routes have since been developed which allow strychnine to be produced efficiently. |
|
 |
Despite its strong bitter taste strychnine was successfully employed by many poisoners wishing to despatch their victims. In 1856 William Palmer was the first Englishman to be convicted of murder by strychnine poisoning and was hanged for his crime. Palmer was a doctor by trade but had financial problems brought about by his love of gambling. One of his friends, John Parsons Cook, owned a race horse and had come into money after it had won a race. After this event Cook experienced pain and peculiar symptoms whenever he drank in Dr Palmer’s presence and died after suffering painful convulsions. It was on this evidence that Palmer was convicted as no strychnine was found in Cook’s corpse. It is thought that the toxicologist failed to extract enough of the poison from the body to obtain a positive result for its presence. This was not likely to have been the first murder that Palmer had committed. Amongst others it is thought he poisoned his mother-in-law, wife and brother. |
Another serial killer who used strychnine was Thomas Neill Cream who acquired the nickname of the ‘Lambeth Poisoner’. Cream was also a doctor and in the early 1890s he murdered four prostitutes with the deadly poison. It was his foolish actions that eventually led to his arrest. After Cream had murdered one of his victims he wrote a letter in an attempt to blackmail the respected Dr William Broadbent using an alias. In the letter Cream stated that he had proof that Broadbent had poisoned the victim, Matilda Clover, but would destroy the evidence for a sum of money. The letter was handed to the police who, until that point, were unaware that Matilda Clover had been murdered. Matilda was an alcoholic and her death was thought to have been brought about by her drinking and as such murder had not been considered. Her remains were exhumed and showed evidence that she had been poisoned. Thus the police had reason to believe that the author of the letter was the poisoner. A New York detective and friend of Dr Cream was in London. He was fascinated by the Lambeth poisonings and Cream had offered to take him on a tour of the area. During the tour Cream described events with such a detailed knowledge that the detective suspected him to be the murderer and reported him to the police. Dr Cream was hanged for his crimes in 1892. |
 |
 |
Despite its poisonous effect strychnine has been used as a medicine, albeit it in very small doses, which is how murderers like Drs Palmer and Cream could obtain the substance. Indeed, Dr Cream is thought to have given his victims strychnine tablets. Strychnine was found in stomach remedies and it was also used as a laxative but it was eventually proved ineffective and withdrawn from use. Strychnine is also known to act as a stimulant in the body and was used as a performance enhancing agent in sport. In the late 1800s cyclists, boxers, swimmers and runners would take strychnine tablets with mixtures of brandy and cocaine as part of their training. Its use was not limited to human athletes only, the very successful Australian racehorse Phar Lap was also fed strychnine, cocaine and other stimulants including caffeine. Perhaps the most famous athlete to use strychnine was Thomas Hicks in the 1904 Olympics. Hicks finished second in the marathon event but was awarded the gold medal when the first place runner was disqualified. During the race Hicks had taken over a milligram of strychnine and a large glass of brandy to help overcome tiredness. Unsurprisingly he collapsed at the end of the race. He recovered although it is thought that just one more dose of strychnine may have killed him. The use of strychnine, like many other stimulants, has now been banned in sport. |
|