Gomberg's discovery
 This is the first example of an organic free radical, discovered by Moses Gomberg [1] in 1900. Three years previously, Gomberg had prepared tetraphenylmethane [2], and he now endeavoured to make hexaphenylethane by the reaction of triphenylmethyl chloride with zinc. To his surprise a yellow hydrocarbon was produced which was not inert as would be expected for hexaphenylethane, but reacted rapidly with oxygen to give triphenylmethyl peroxide and with halogens to give the triphenylmethyl halide. Gomberg rejected the idea of a specially reactive hexaphenylethane molecule, and postulated the formation of the triphenylmethyl radical.
This is the first example of an organic free radical, discovered by Moses Gomberg [1] in 1900. Three years previously, Gomberg had prepared tetraphenylmethane [2], and he now endeavoured to make hexaphenylethane by the reaction of triphenylmethyl chloride with zinc. To his surprise a yellow hydrocarbon was produced which was not inert as would be expected for hexaphenylethane, but reacted rapidly with oxygen to give triphenylmethyl peroxide and with halogens to give the triphenylmethyl halide. Gomberg rejected the idea of a specially reactive hexaphenylethane molecule, and postulated the formation of the triphenylmethyl radical.
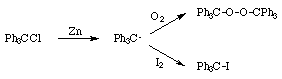
The yellow colour of the triphenylmethyl radical in solution becomes more intense on heating and less intense on cooling: a reversible equilibrium of the triphenylmethyl radical with its dimer was postulated [3].
References
1. M. Gomberg, Ber., 1900, 33, 3150; J. Amer. Chem. Soc., 1900, 22, 757.
2. M. Gomberg, Ber., 1897, 30, 2043; J. Amer. Chem. Soc., 1897, 20, 773.
Some contemporary reviews
Back to main menu


