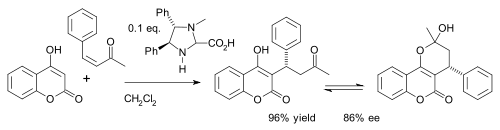
Preparation of Coumarins: the Pechmann Condensation
In 1883 Hans von Pechmann and Carl Duisberg {H. v Pechmann, and C. Duisberg, Ber., 1883, 16, 2119} found that phenols condense with beta-ketonic esters in the presence of sulphuric acid, giving coumarin derivatives.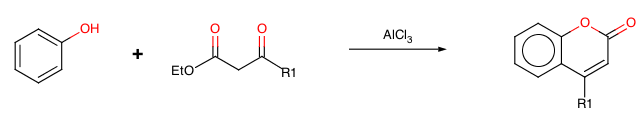
With R1=OH we have 4-hydroxycoumarin, the starting material for the preparation of Warfarin
The reaction is also catalysed by the presence of a Lewis acid such
aluminium(III) chloride or other strong Brönstedt
acids such as methanesulphonic acid to form a coumarin. The acid catalyses
trans-esterification as well as keto-enol tautomerisation.
Bismuth(III) chloride, also a Pechmann catalyst, provides a recent procedure
for 4-substituted coumarins.{
An Efficient and Practical Procedure for the Synthesis of 4-Substituted Coumarins
Surya K. De*, Richard A. Gibbs, Synthesis, 2005, 1231.}
In another Pechmann condensation synthesis, the ionic liquid 1-butyl-3-methylimidazolium chloroaluminate
([bmim]Cl.2AlCl3) plays the dual role of solvent and Lewis acid catalyst
for the reaction of phenols with ethyl acetoacetate leading to coumarin derivatives.
Here, the reaction time is reduced drastically even at ambient conditions.
{M. K. Potdar, S. S. Mohile, M. M. Salunkhe, Tetrahedron Lett., 2001,
42, 9285}
Solid acid catalysts with the H+ attached to the polymer surface such as
Nafion 417 or Amberlyst IR120 can be used. Thus resorcinol
reacts with ethyl acetoacetate in boiling toluene in the presence of Nafion sheet
to form the coumarin 7-hydroxy-4-methylcoumarin.
This preparation forms the basis of a student organic chemistry experiment at
Penn State University. In this case the coumarin,
{also named, 7-hydroxy-4-methyl-2H-benzo[b]-pyran-2-one} is not a blood thinner but is a drug
used in bile therapy,
Hymecromone.
The material is also, in highly purified form a laser dye, and the starting material
for some insecticides!

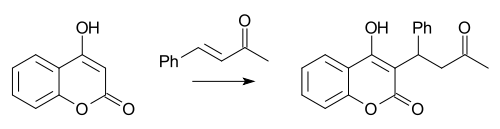 Reaction of 4-hydroxycoumarin with benzylacetone under
Reaction of 4-hydroxycoumarin with benzylacetone under