 Deposition of Ultrananocrystalline Diamond (UNCD) films
Deposition of Ultrananocrystalline Diamond (UNCD) films
We spent a few years investigating the deposition and properties of so-called ultra-nanocrystalline (UNCD) diamond films. These are diamond films where the grain size is around 2-5 nm in size, and have wide grain boundaries. Such films have potential for use in electronics, MEMS or as biochemical sensors, since they combine many of the superb properties of diamond with a smoothness on the nm scale. These films were first developed by Argonne National Labs in the US, who used a novel Ar/CH4/H2 gas mixture to make them. We investigated the gas chemistry occurring during deposition of these films to try to identify the growth mechanism. In particular, we were interested in ascertaining the identity of the growth species, and to understand why the crystallites continually renucleate.
 |
 |
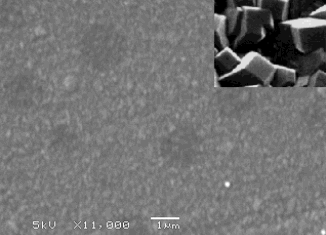
| An SEM photo of a UNCD film grown in a microwave CVD reactor. Inset is an SEM image of a microcrystalline film taken at the same magnification for comparison. |
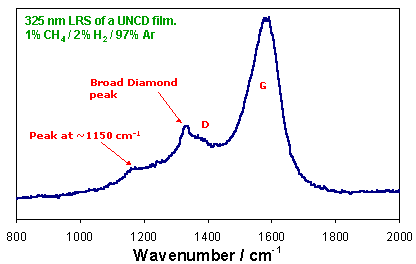
UV laser Raman spectrum from a UNCD film. The peak at ~1150 cm-1 is associated with sp2-carbon species at the grain boundaries. |
We developed a model for UNCD growth which involves a competition for the surface radical sites by CH3 and C atoms. The C2 radical has been discounted as a growth precursor because its concentration at the substrate surface is many orders of magnitude too small to be important in growth (10,000× less than that of CH3). In our model, gas-phase H atoms produce plenty of surface radicals by H-abstraction reactions, and CH3 is the dominant hydrocarbon radical adding to these surface sites - which is why UNCD films are predominantly diamond. However, under the typical UNCD growth conditions (high Ar concentration, >100 Torr), C atoms (and, to a lesser extent, other reactive C1 species) can survive long enough to reach the growing surface in sufficient concentration to affect growth. If a C atom adds to the surface, it can either hydrogenate to CH3 (in which case we are back to normal diamond growth), or sometimes cross-link, bridge, or restructure on the surface to form a defect. This defect would break the symmetry of the lattice and so act as a renucleation site. The ratio of the rates of addition of CH3 to that of C determines the renucleation rate, and therefore the size of the crystallites, and therefore whether the films are MCD, NCD, or even UNCD.
Related Papers
- P.W. May, J.A. Smith, Yu. A. Mankelevich, "Deposition of NCD Films Using Hot Filament CVD and Ar/CH4/H2 Gas Mixtures", Diam. Relat. Maters. 15 (2006) 345-352.

- P.W. May, J.N. Harvey, J.A. Smith, Yu. A. Mankelevich, "Re-evaluation of the mechanism of ultrananocrystalline diamond deposition from Ar/CH4/H2 gas mixtures", J. Appl. Phys. 99 (2006) 104907.

- P.W. May, Yu. A. Mankelevich, "Experiment and modeling of the deposition of ultrananocrystalline diamond films using hot filament chemical vapor deposition and Ar/CH4/H2 gas mixtures: A generalized mechanism for ultrananocrystalline diamond growth", J. Appl. Phys. 100 (2006) 024301.

