|
pH |
||
| WATER The Water Cycle Water in a Pool Water compared Pool Processes POOL PROCESSES CHEMISTRY IN OZONE DISINFECTION NEW IDEAS Hannah Morgan, University of Bristol, School of Chemistry, hm9921@bris.ac.uk |
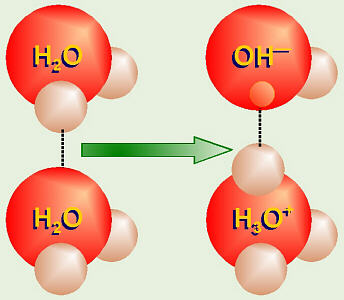
All the equilibria in the pool water chemistry are pH controlled. pH is a measure of the hydrogen ion concentration. The following diagram shows how water molecules dissociate into ions, the ions are a measure of how acid or alkali the solution is.
In pool water disinfection, the important equation takes hypochorus acid to hypochlorite acid. Hence, if the pH rises, the hydrogen ion concentration rises, and the equilibrium goes to the left, promoting formation of hypochlorus acid, and decreasing the amount of hypochlorite acid. Hypochlorus acid is the stronger acid, so a high pH can severely reduce disinfecting properties of chlorine, although free chlorine is kept relatively low. The addition of chlorine gas will reduce the pH, the addition of sodium hypochlorite will increase it. Most disinfectants generally work best at low pH. Coagulants also work best at a certain pH, Aluminium ones work best at 6.5 - 7.2 whereas iron based coagulants work best at 7.2 - 7.8. |