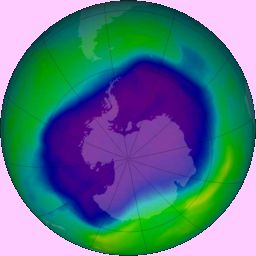
The hole in ozone layer over the Antarctic.
Photo: NASA (via Wikimedia Commons) |

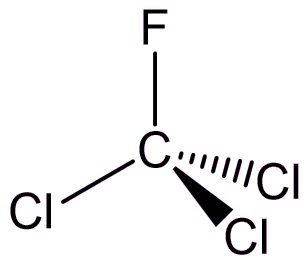
Trichlorofluoromethane
CFCl3 (or CFC-11)
The ozone-eliminating gas
that we thought had gone for good...

Simon Cotton
University of Birmingham

Molecule of the Month June 2019
Also available: HTML version.

|
|
Looks like chloroform...
Indeed it does (see MOTM for October 2006), with a fluorine substituted for a hydrogen, but it’s not used as an anaesthetic.
What is it used for, then?
Particularly in refrigerators and air-conditioning systems, as well as a blowing agent in plastic manufacture, notably polyurethane foam.
How do you make CFCl3?
It’s harder than making chloroform. The method was devised by the Belgian Chemist Fredéric Swarts (1866-1940), who was responsible for many breakthroughs in the area. He found that you could use the reaction between SbF3Br2 (formed in situ from SbF3 with Br2) with CCl4 (though CF2Cl2 is often obtained as a co-product). Indeed, this became known as the Swarts reaction. It seems to have been used by Midgley to make CFCs in the 1920s.
SbF3Br2 + CCl4 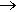 CFCl3 + SbF2Br2Cl
CFCl3 + SbF2Br2Cl
Other reactions are possible; HF and CCl4 give a mixture of chlorofluorocarbons, and XeF2 reacts with CHCl3 forming a mixture largely of CFCl3 and CHFCl2.
So why is it in the news?
Because it is getting into the atmosphere, and it is not supposed to.
Explain, please.
It is a bit of a long story, and it goes back to refrigerators.
Why are these chemicals involved with refrigerators?
Well, once upon a time (circa 1930), hardly anyone had a refrigerator, a.k.a. a fridge. You either ate up your food quickly, before it could go off, or found a way of preserving it. The Royal Navy made a big thing of salt beef. Some bought blocks of ice to pack around food. And in the winter, snow drifts might come in handy. A refrigerator extended the life of food; a very healthy thing, too.
A refrigerator contains a closed system full of a refrigerant, or coolant (see diagram, right).
It contains a continuous piece of pipework linking a set of ‘condenser coils’ on the exterior or the fridge with a set of ‘evaporator coils’ inside, so that the refrigerant fluid can make a complete circuit between the two. Start with the vaporised refrigerant entering the compressor (4); this compresses (and thus warms) the vapour, pushing it through the exterior ‘condenser coils’ (1), where it is cooled down by the exterior air (the large area of the coils promotes the cooling effect) so the gas cools sufficiently to liquefy and passes along the tubing (2) through an expansion valve. The sudden expansion here causes the liquid to evaporate and cool down sharply in the process, so that cold refrigerant vapour passes through the ‘evaporator coils’(3). This cold vapour ensures that the fridge is cold. It absorbs heat from the coils and continues circulating back to the compressor (4), and goes through the cycle once again. |
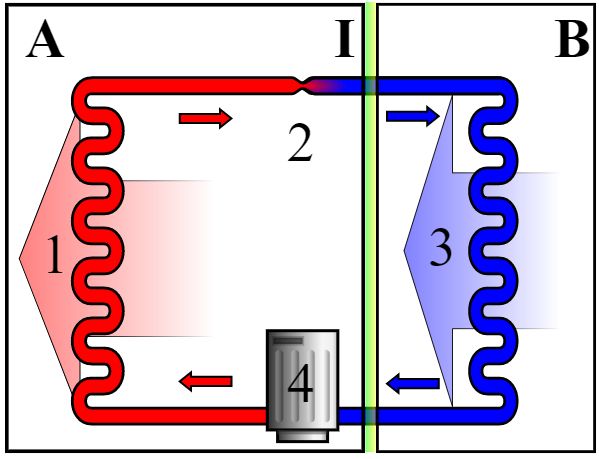
Schematic diagram of the operation of a fridge.
Image: Keno at en.wikipedia.
|
So CFCl3 was the obvious choice?
Not at first, no. Back in the 1920s, there was a problem with refrigerators. In those days, fridges used refrigerants that were toxic, such as ammonia (NH3), chloromethane (CH3Cl) and sulfur dioxide (SO2). If there was a leakage, people could be killed, and in the 1920s a number of fatalities were caused by chloromethane. Alternatives like butane were very flammable, so companies looked for new alternative refrigerants. People started to keep their fridges out of doors.
In 1928, an engineer named Thomas Midgley, Jr., who had already come up with tetraethyllead (MOTM January 2001) as an anti-knock additive for petrol, looked at the properties of chlorofluorocarbons (which many years later came to be known as CFCs), a family that came to be generally known as ‘Freon’.
These seemed ideal refrigerant coolants, a 1932 publication listed the properties of CF2Cl2, typical of the family: 1. It is non-inflammable. 2. It does not support combustion. 3. It is nonexplosive. 4. It is non-irritating. 5. It is nontoxic. 6. It has no odour even in fairly high concentrations. 7. Its products of decomposition give ample warning. 8. It is stable. 9. It is noncorrosive. 10. Leaks can be readily detected. 11. It is not absorbed by foods or materials being refrigerated. 12. It has no effect on flowers, fruits, vegetables, dairy products, or furs, or materials being refrigerated.
The boiling point of CFCl3, 23.77°C (296.92 K) made it ideally suited to use in refrigerating systems.
So why is there a problem now?
That’s all to do with the ozone layer. This is the part of the stratosphere, between about 15 and 30 km above the Earth’s surface, where ozone is formed when UV radiation from the sun interacts with atmospheric O2 (dioxygen) molecules, turning them into O3, ozone. The ozone has an important role as a filter for UV radiation, preventing most of it from reaching the earth and contributing to illnesses like skin cancer.
As the production and use of CFCs spread (at levels in excess of 300,000 tonnes a year), they became more abundant in the atmosphere. In 1973 the British eco-guru (and chemist) James Lovelock reported detecting CFCl3 along with other halogenated organic molecules over the North Atlantic, and the following year Molina and Rowland’s pioneering study was published showing that Cl atoms, formed by dissociation of molecules like CFCl3, could readily catalyse the decomposition of ozone.
CFCl3  CFCl2 + Cl CFCl2 + Cl
Then Cl + O3  ClO + O2 ClO + O2
And ClO + O  Cl + O2 Cl + O2
The seriousness of this discovery was that just one free chlorine atom, a very reactive free radical, could go on to destroy 100,000 molecules of ozone.
|
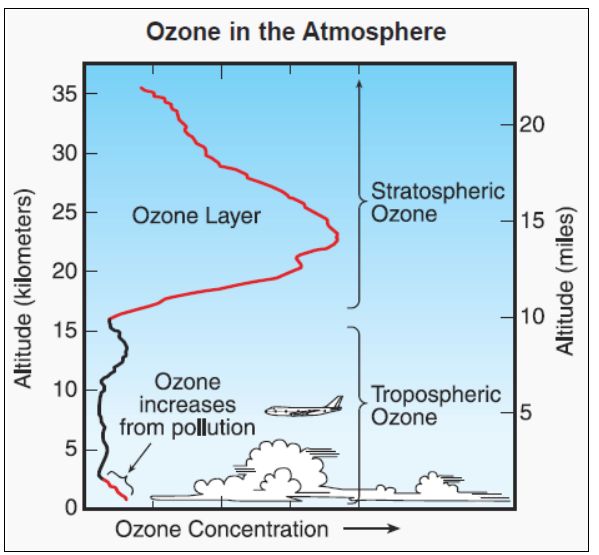
Ozone in the atmosphere.
Image: http://www.theozonehole.com/ |
During the Antarctic spring, from September to November, a seasonal depletion of ozone occurs in the polar region, caused by this reaction, resulting in what is known as the ‘ozone hole’. Then in 1985, Farman, Gardiner and Shanklin, three British Atlantic Survey scientists, reported that – unlike the case 20 years earlier – severe depletions were taking place. These depletions at around 30% came as a shock. For once World politicians could agree on something, and most nations, including the chemical producers, signed up in 1987 to the global treaty known as the Montreal Protocol, agreeing to reduce levels of ozone-depleting substances, subsequently agreeing to eliminate CFC production completely. The seasonal ozone hole went on getting bigger for some years, but has now stabilised and is projected to get back to earlier levels by the late 21st century (!)
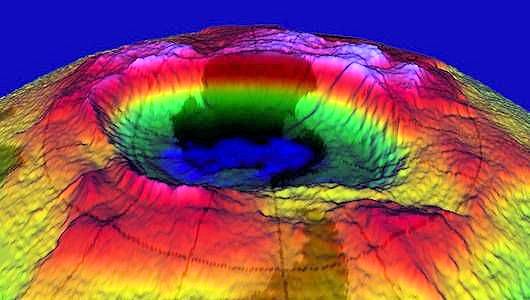
A 3-D image of the ozone hole over Antarctica, produced with a mapping spectrometer.
Photo: NASA
So what is the problem then?
Well, things seemed to be going well, as in 2014 there was evidence that not only were atmospheric CFCl3 levels dropping back, the ozone layer hadn’t just stabilised but had actually started to thicken up slightly. Then in people who had been monitoring CFC levels noticed that the rate of fall in CFCl3 levels had started to fall off.
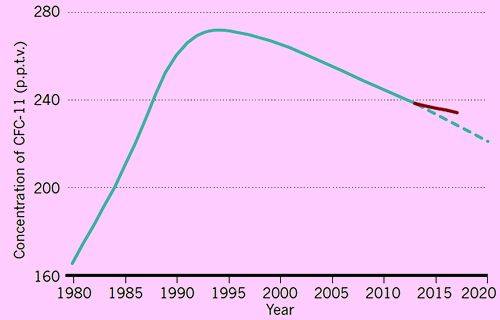
The sudden, unexpected rise in CFC-11 in the atmosphere.
(from M. I. Hegglin, Nature, 2018, 557, 317-318).
So why is that?
Background probes by the Environmental Investigations Agency (EIA) discovered that CFC-11 was still widely available and freely used in China, even though CFCl3 has been completely banned in all developing nations – which includes China – since 2010. CFC-11 isn’t just a refrigerant substance, it makes a very efficient ‘blowing agent’ for the expanded polystyrene foam that is used to make thermal insulation by Chinese builders, who prefer it to alternatives. In May 2019 a report in Nature by a team from Bristol University showed that emissions were definitely originating in East Asia, almost certainly the provinces of Shandong and Hebei in the north-east. Monitoring stations in Japan and South Korea detected the gas, and then knowledge of the wind directions were used to trace its origins back into Asia.
Does it matter?
Most definitely it does. It amounts to some 7,000 tonnes of CFCl3 being released into the atmosphere each year (and getting bigger). Chinese authorities say that the illegal CFC emissions came from 'rogue factories'. These factories should be using other substances like hydrofluorocarbons - HFCs - which do not damage the ozone layer (though they are big greenhouse gases). If the Chinese government successfully track down those people illegally using CFCl3 and drive it out of use, then that is good news, but China is a huge country and they have a big task on their hands. We do not know why the Chinese authorities did not pick up this problem earlier. Another issue is that, in addition to the building firms using it, there are some companies actually making CFC-11.
|
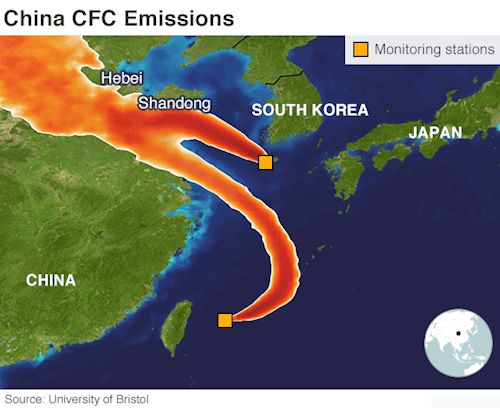
The CFC-11 emissions were monitored and traced back to China.
Image; University of Bristol |
And if it is not resolved?
The Montreal protocol is the best example of the nations of the world cooperating to the benefit of the environment, a fantastic success story. This could delay the healing of the hole in the ozone layer by many years – or something worse.

Bibliography
- Chapman and Hall Combined Chemical Dictionary code number: DLK32-M
Synthesis of CFCl3
- F. Swarts, Bull. Acad. Roy. Belg., 1892, 24, 309-320, 474-484.
- O. Ruff et al., Z. Anorg. Allg. Chem., 1931, 201, 245 (synth)
- K. O. Christie et al., J. Org. Chem., 1964, 29, 3007 (synth)
- W. W. Dukat, J. H. Holloway, E. G. Hope, P. J. Townson and R. L. Powell, J. Fluorine Chem. 1993, 62, 293-296; M. M. Shaw, R. G. Smith, C. A. Ramsden, J. Fluorine Chem., 2002, 116, 71–73.
- R. E. Banks, D. W. A. Sharp and J. C. Tatlow, Fluorine - the first hundred years (1886–1986), Elsevier Sequoia, Lausanne and New York, 1986.
CFCs and ozone layer destruction
- J. E. Lovelock, R. J. Maggs and R. J. Wade, Nature, 1973, 241, 194–196
- M. J. Molina and F. S. Rowland, Nature, 1974, 249, 810–812.
- J. C. Farman, B. G. Gardiner and J. D. Shanklin, Nature, 1985, 315, 207-210.
- S. Elliott and F. S. Rowland, J. Chem. Educ. 1987, 64, 387-391.
- http://www.theozonehole.com/cfc.htm
- http://www.meti.go.jp/policy/chemical_management/ozone/files/pamplet/panel/08e_basic.pdf
Refrigerants
- T. Midgley and A. L. Henne, Ind. Eng. Chem., 1930, 22, 542- 545 (organic fluorides as refrigerants)
- R. J. Thompson, Ind. Eng. Chem., 1932, 24, 620-623 (freons as refrigerants)
- T. Midgley, Ind. Eng. Chem., 1937, 29, 241–244 (Midgley’s story of the thinking behind CFCs)
- K. R. Williams, J. Chem. Educ., 2000, 77, 1540-1541 (‘refrigeration—from Ice man to ozone hole’)
- S. Benhadid-Dib and A. Benzaoui, Energy Procedia, 2011, 6, 347–352; 2012, 14, 807 – 816 (refrigerants and their impact in the environment)
Midgley
- T. Midgley, From the Periodic Table to Production: The Life of Thomas Midgley, Jr., the Inventor of Ethyl Gasoline and Freon Refrigerants, Stargazer Publishing, 2001.
- C. J. Giunta, Bull. Hist. Chem., 2006, 31, 66-74. (‘Thomas Midgley Jr. and the Invention of Chlorofluorocarbon Refrigerants’)
- H. E. B. Viana and P. A. Porto, J. Chem. Educ., 2013, 90, 1632–1638 (Thomas Midgley, Jr., and CFCs)
New CFC-11 emissions
- A. McCulloch, P. Ashford and P.M. Midgley, Atmos. Env., 2001, 35, 4387–4397 (historic emissions of fluorotrichloromethane (CFC-11) based on a market survey)
- D. Wan, J. Xu, J. Zhang, X. Tong and J. Hu, Atmos. Env., 2009, 43, 5822–5829 (historical and projected emissions of major halocarbons in China)
- M. I. Hegglin, Nature, 2018, 557, 317-318 (‘Increased emissions of ozone depleters’)
- S. A. Montzka, et al, Nature, 2018, 557, 413–417 (‘an unexpected and persistent increase in global emissions of ozone-depleting CFC-11’)
- M. F. Lunt, et al., Geophys. Res. Lett., 2018, 45, 11423–11430 (‘Continued emissions of the ozone-depleting substance carbon tetrachloride from eastern Asia’)
- Y. Lin, D. Gong, S. Lv, Y. Ding, G. Wu, H. Wang, Y. Li, Y. Wang, L. Zhou and B. Wang, Environ. Sci. Technol. Lett., 2019, 6, 114−118 (high levels of CFC-11 ‘at a remote mountain-top site in southern China’)
- M. Rigby et al., Nature, 2019, 569, 546-550 (‘Increase in CFC-11 emissions from eastern China based on atmospheric observations’)
- Environmental Investigation Agency (EIA) Report, July 2018 (with downloadable pdf)
- https://www.chemistryworld.com/opinion/the-china-cfc-dilemma/3009280.article (‘the China CFC dilemma’)
- https://www.nytimes.com/2018/11/03/climate/china-ozone-cfcs.html
- https://chemlinked.com/news/chemical-news/china-destroys-cfc-11-illegal-manufacturing-facility
- https://chemicalwatch.com/71603/china-arrests-three-suspects-over-illegal-cfc-11-use (arrests in China)


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.8194415]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.8194415]

![]()
![]()
![]()
![]()

 CFCl3 + SbF2Br2Cl
CFCl3 + SbF2Br2Cl



