![]()
Chlorophyll
The green colour of plants
![]()
![]()
Molecule of the Month May 2000
(Updated July 2017)
Also available: JSMol version.
![]()

Aerial view of the Amazon rainforest.
ChlorophyllThe green colour of plants
Molecule of the Month May 2000
|
 Aerial view of the Amazon rainforest. |
Yes, but it’s much more than just a pigment. Chlorophyll is the molecule that absorbs sunlight and uses its energy to synthesise carbohydrates from CO2 and water. This process is known as photosynthesis and is the basis for sustaining the life processes of all plants. Since animals and humans obtain their food supply by eating plants, photosynthesis can be said to be the source of our life also.
Effectively, yes.
A whole bunch of bewigged European scientists solved the puzzle piece by piece over a period of about 100 years. In 1780, the English chemist Joseph Priestley found that plants could "restore air which has been injured by the burning of candles." He used a mint plant, and placed it into an upturned glass jar in a vessel of water for several days. He then found that "the air would neither extinguish a candle, nor was it all inconvenient to a mouse which I put into it". In other words, he discovered that plants produce oxygen (luckily for the mouse!).
 |
 |
 |
| Joseph Priestley | Antoine Lavoisier | Jan Ingenhousz |
A few years later, in 1794, the French chemist Antoine Lavoisier, discovered the concept of oxidation, but before he could make any further advances he was executed during the French Revolution for being a Monarchist sympathiser. The judge who pronounced sentence said "The Republic has no need for scientists". So it fell to a Dutchman, Jan Ingenhousz, who was court physician to the Austrian empress, to make the next major contribution to the mechanism of photosynthesis. He had heard of Priestley's experiments, and a few years later spent a summer near London doing over 500 experiments, in which he discovered that light plays a major role in photosynthesis.
"I observed that plants not only have the faculty to correct bad air in six to ten days, by growing in it...but that they perform this important office in a complete manner in a few hours; that this wonderful operation is by no means owing to the vegetation of the plant, but to the influence of light of the sun upon the plant."
 |
 |
 |
| Jean Senebier | Theodore de Saussure | Julius Robert Mayer |
Very soon after, more pieces of the puzzle were found by two chemists working in Geneva. Jean Senebier, a Swiss pastor, found that "fixed air" (CO2) was taken up during photosynthesis, and Theodore de Saussure discovered that the other reactant necessary was water. The final contribution to the story came from a German surgeon, Julius Robert Mayer, who recognised that plants convert solar energy into chemical energy. He said:
"Nature has put itself the problem of how to catch in flight light streaming to the Earth, and to store the most elusive of all powers in rigid form. The plants take in one form of power, light; and produce another power, chemical difference."
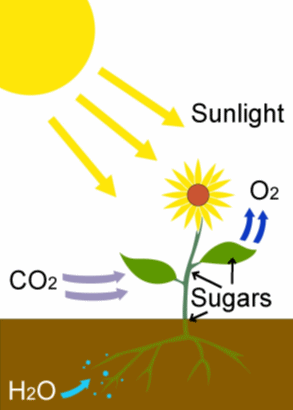 The actual chemical equation which takes place is the reaction between carbon dioxide and water, catalysed by sunlight, to produce glucose and a waste product, oxygen. The glucose sugar is either directly used as an energy source by the plant for metabolism or growth, or is polymerized to form starch, so it can be stored until needed (see MOTM page on glucose). The waste oxygen is excreted into the atmosphere, where it is utilized by plants and animals for respiration. The chemical equation for photosynthesis is:
The actual chemical equation which takes place is the reaction between carbon dioxide and water, catalysed by sunlight, to produce glucose and a waste product, oxygen. The glucose sugar is either directly used as an energy source by the plant for metabolism or growth, or is polymerized to form starch, so it can be stored until needed (see MOTM page on glucose). The waste oxygen is excreted into the atmosphere, where it is utilized by plants and animals for respiration. The chemical equation for photosynthesis is:
6 CO2 + 6H2O ![]() C6H12O6 + 6 O2
C6H12O6 + 6 O2
where C6H12O6 is glucose, with sunlight being the energy source and chlorophyll the catalyst.
Chlorophyll is the photoreceptor molecule that traps this ‘most elusive of all powers’. It is found in the chloroplasts of green plants, and is what makes green plants, green. Its name actually comes from the Greek chloros meaning ‘green’, and phyllon meaning ‘leaf’. The basic structure of a chlorophyll molecule is a porphyrin ring (a conjugated flat ring-shaped molecule based on porphene), co-ordinated to a central metal atom. This is very similar in structure to Vitamin B12 and the heme group found in hemoglobin, except that in Vitamin B12 the central atom is cobalt, in heme it’s iron (see page on Hemoglobin), whereas in chlorophyll it is magnesium.
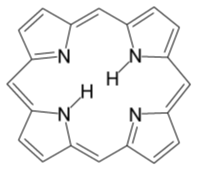 |
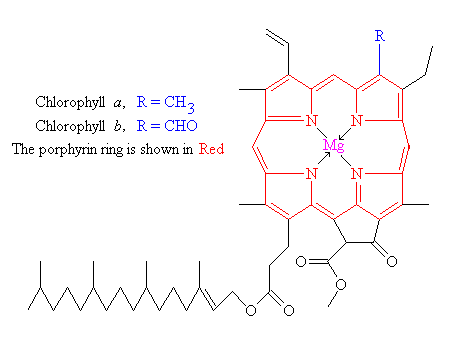 |
| Porphene | Chlorophyll |
There are actually several types of chlorophyll, which differ only slightly in the composition of a side-chain. The two most common types are chlorophyll a where the side-group is -CH3, and chlorophyll b where it is CHO. Both of these two chlorophylls are very effective photoreceptors because they contain a network of alternating single and double bonds, and the orbitals can delocalise, stabilising the structure. Such delocalised polyenes have very strong absorption bands in the visible regions of the spectrum, allowing the plant to absorb the energy from sunlight.
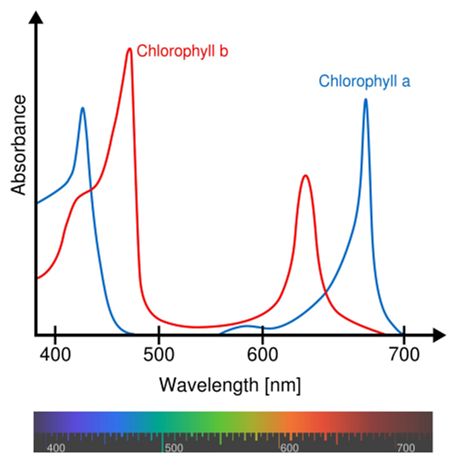
Absorption spectra of chlorophyll a and b.
 The different side-groups in the 2 chlorophylls 'tune' the absorption spectrum to slightly different wavelengths, so that light that is not significantly absorbed by chlorophyll a, at, say, 460 nm, will instead be captured by chlorophyll b, which absorbs strongly at that wavelength. Thus these two kinds of chlorophyll complement each other in absorbing sunlight. Plants can obtain all their energy requirements from the blue and red parts of the spectrum; however, there is still a large spectral region, between 500-600 nm, where very little light is absorbed. This light is in the green region of the spectrum, and since it is reflected, this is the reason plants appear green. In fact, chlorophyll a is used as a green dye (called ‘Natural Green 3’) that’s often found in soaps and cosmetics.
The different side-groups in the 2 chlorophylls 'tune' the absorption spectrum to slightly different wavelengths, so that light that is not significantly absorbed by chlorophyll a, at, say, 460 nm, will instead be captured by chlorophyll b, which absorbs strongly at that wavelength. Thus these two kinds of chlorophyll complement each other in absorbing sunlight. Plants can obtain all their energy requirements from the blue and red parts of the spectrum; however, there is still a large spectral region, between 500-600 nm, where very little light is absorbed. This light is in the green region of the spectrum, and since it is reflected, this is the reason plants appear green. In fact, chlorophyll a is used as a green dye (called ‘Natural Green 3’) that’s often found in soaps and cosmetics.
Good question – a black pigment would be much more efficient because all the visible light energy from the sun would be absorbed, rather than some being reflected back and wasted, as the green light is at present. Scientists are still unclear about the reasons for this. One idea is that because all modern plants are believed to have evolved from a common ancestor - a sort of green algae – they all inherited the same chlorophyll. If this molecule did its job of absorbing light well enough, there would be no need to evolve a more efficient version. Other reasons state that evolution is not a directed process, and is not capable of thinking like an engineer. Given the same problem, an engineer might design a photoreceptive molecule that absorbs as much of the spectrum as possible, including ultraviolet and infrared (i.e. a black pigment). But evolution can only work with what it has, and slowly change the current system into a better version. If there’s no driving need for a better version, then the molecule won’t evolve. Even if there is a need, there may not be an evolutionary mechanism to achieve it. This is a good argument to use on Creationists – a truly ‘Intelligent Designer’ would not have chosen an inefficient molecule such as chlorophyll to harvest solar energy; a far better choice would have been a molecule such as retinal (see MOTM page on carotene) which absorbs much more of the spectrum.
Chlorophyll is not the only pigment in leaves. But chlorophyll absorbs so strongly that it can mask other less intense colours. Some of these more delicate colours (from molecules such as carotene and quercetin) are revealed when the chlorophyll molecule decays in the autumn, and the woodlands turn red, orange, and golden brown. Chlorophyll can also be damaged when vegetation is cooked, since the central magnesium atom is replaced by hydrogen ions. This affects the energy levels within the molecule, causing its absorbance spectrum to alter. Thus, cooked leaves change colour - often becoming a paler, insipid yellowy green.



As the chlorophyll in leaves decays in the autumn,
the green colour fades and is replaced by the oranges and reds of carotenoids.
The chlorophyll molecule is the active part that absorbs the sunlight, but just as with heme (see page on Hemoglobin), in order to do its job (synthesising carbohydrates) it needs to be attached to the backbone of a very complicated protein. This protein may look haphazard in design, but it has exactly the correct structure to orient the chlorophyll molecules in the optimal position to enable them to function efficiently manner.
In fact the energy absorbed by one chlorophyll molecule is not sufficient to catalyse the reaction to make carbohydrates. So, many chlorophyll molecules work together, like a big radar dish, to collect light energy and funnel it to a central ‘master’ chlorophyll molecule by way of a series of electron-transfer processes. With time, the master chlorophyll molecule becomes more and more positively charged. Once the master chlorophyll has stored up sufficient charge, it can neutralise itself by grabbing an electron from a nearby water molecule: the water is thus oxidised to O2 gas and H+ ions. The H+ ions then diffuse through the cell membrane and in doing so set up a potential difference. An enzyme called ATP synthase then collects the H+ ions and shuttles them back across the membrane to where they came from, and the potential energy that’s released is used by the enzyme to make ATP molecules from ADP (see MOTM page on ATP). ATP is the molecule that stores energy for short-term use in plants and animals. The ATP is transported around the plant to where the energy is needed, such as places where the plant is growing, and other enzymes then use the energy stored in the ATP to fix CO2 into sugars in a complicated process called the Calvin Cycle. The sugars are either oxidised immediately to release their energy for various metabolic processes or polymerized into starch (a long-term energy store) or cellulose which makes up the structure of the plant cells.
 So you could say that plants are made up of solid carbon dioxide?
So you could say that plants are made up of solid carbon dioxide?Yes, pretty much. It’s amazing to think that when you look at plants, all the leaves, stems, tree bark, even the fruit and nuts, were once just carbon dioxide and water. This is becoming more important when we consider the effect that atmospheric CO2 has upon global warming. One of the best ways to ‘mop up’ CO2 gas is to fix it into plants. The amount of CO2 removed from the atmosphere each year by photosynthetic organisms is enormous – estimates suggest something like one billion tonnes of carbon per year. Green plants and algae help to mitigate the excess CO2 in the atmosphere put there by mankind burning fossil fuels. So deforestation, which is occurring all over the Earth, is one of the worst things we can do if we want to prevent CO2 build up and a possible runaway Greenhouse Effect. The second worst thing we can do is to burn those forests, releasing the stored CO2 back into the air – but guess what - we’re doing that too!
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245597]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245597]