|
Origins
It
was discovered by world-reknowned, French scientist Louis Pasteur in
the 1860s through his research on the fermentation of beer and ale. He
determined yeast was alive and actively reproduced. He learned the
compound, now referred to as diacetyl, is naturally produced through the
fermentation process.
 Pasteur
at work in his laboratory, a painting by Albert Edelfelt in 1885.
European
chemists later synthesized diacetyl from methyl ethyl ketone in
the 1920s and shortly after, artificial-butter flavoring spread rapidly.
Role of Diacetyl in Brewing Yeast
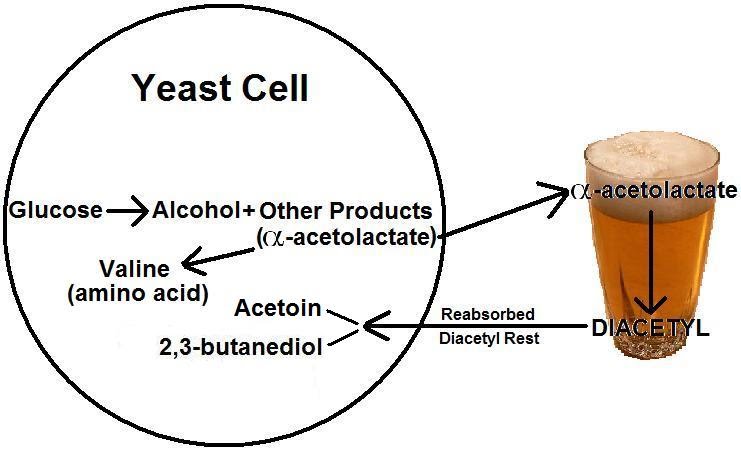
are single-celled microorganisms that reproduce by budding. In beer,
they are responsible for converting sugar into alcohol, as well as other
by-products. It is this conversion that provides the main source of
diacetyl in beer. Outside of alcohol and carbon dioxide, many
other products occur from this conversion including the ester, α-acetolactate. Some of
this ester is used by the cell to produce the amino acid
valine, but most is dumped into your beer. Oxygen
and high temperatures help transform this into diacetyl. The
amount of diacetyl wanted varies from the type of beverage being brewed
and if taken, the next step helps determine this. After
fermentation, the yeast will reabsorb as much or as little diacetyl
intended. Often this is referred to as a "diacetyl rest," in
which high temperatures, and allowing the beer to remain in the
fermenter after fermentation, will cause the yeast to reabsorb
excess diacetyl and break it down.
At
low levels, diacetyl contributes a "slickness" on the tongue. As
the amount increases, it helps give a buttery or butterscotch taste to
the beer. Finding the proper medium is essential in creating the most
desirable beverage.
|
Originates from Beer |