

 |
 |
Booker-Milburn Group |
|---|
| Research |
|---|
Natural Product Total Synthesis
|
||||
|---|---|---|---|---|
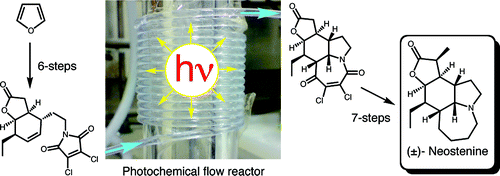
A particular focus of the group is research into the total synthesis of natural products in particular that of the stemona alkaloids. In 2008 we reported an efficient total synthesis of neostenine which utilised a [5 + 2] photocycloaddition of maleimides that had been developed during our photochemical work. We are currently working on a second generation synthesis of neostenine, but the original synthesis is outlined below (1).
|
||||
 |
||||
The initial product of the Diels-Alder cycloaddition between furan and fumaryl chloride was reduced in situ to diol (±)-19 using Paquette's procedure (2). Reaction of 19 with 2 equivilents of MsCl followed by displacement with KCN gave dinitrile 20. Basic hydrolysis of 20 gave the diacid 18. Acid catalysed bislactonisation was then achieved with yield > 90% to give 17.
After optimisation it was found that portionwise addition of 17 to a solution of EtMgCl/CuBr •DMS (3 equiv) at -20 °C gave 16 in 94% yield. The carboxylic acid moiety of 16 could be selectively reduced by conversion to the mixed anhydride with ethyl chloroformate followed by reduction with sodium borohydride (3). A Mitsunobu reaction with dichloromaleimide gave 22. By using our novel flow reactor we could obtain multigram quantities of 23 in good yield. The use of this flow reactor was instrumental in the viability of this route to neostenine as for the first time it enabled gram quantities of 23 to be produced quickly (1.3 g in 9 h). Following this [5 + 2] photocycloaddition the completion of the neostenine total synthesis could be undertaken. Dechlorination of 23 with Zn/AcOH gave 24 which could be selectively reduced to 25 using LiAl(OtBu)3H (4). Conversion to the thiocarbonate 28 and then treatment under standard Bu3SnH/AIBN conditions gave the deoxygenated lactone-amide 29. Methylation of 29 and then epimerisation gave oxoneostenine 30 as a 5:1 mixture of α/β-epimers. Finally 30 was selectively reduced with RhH(CO)(PPh3)3/Ph2SiH2 (5) to give neostenine in 69% yield. |
||||
References (1): A Protecting Group Free Total Synthesis of (±)-Neostenine via the [5 + 2] Photocycloaddition of Maleimides; Michael D. Lainchbury, Marcus I. Medley, Piers M. Taylor, Paul Hirst, Wolfgang Dohle, Kevin I. Booker-Milburn J. Org. Chem. 2008, 73, 6497-6505. DOI. (2): Paquette, L. A.; Kravetz, T. M.; Charumilind, P. Tetrahedron 1986, 42, 1779. (3): N'Zoutani, M. A.; Pancrazi, A.; Ardisson, J. Synlett 2001, 769. (4): Danishefsky, S.; Schuda, P. F.; Kitahara, T.; Etheredge, S. J. J. Am. Chem. Soc. 1977, 99, 6066. (5): Kuwano, R.; Takahashi, M.; Ito, Y. Tetrahedron. Lett. 1998, 39, 1017. |
||||
| Professor Kevin Booker-Milburn, School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. |
|
E-mail: k.booker-milburn@bristol.ac.uk Tel: +44 (0)117 9288157 Lab Tel: +44 (0)117 9546320 Website Built and Maintained by Steve Cooper |
|---|