CVD Diamond - a New Technology for the Future?
CVD Diamond - a New Technology for the Future?
School of Chemistry, University of Bristol,
Bristol BS8 1TS, U.K.
[This is a copy of an article which appeared in Endeavour Magazine 19(3), (1995) pp101-106. (© copyright Elsevier, 1995)]
1 Introduction
Diamond has long held a special place in the hearts and minds both of scientists and the public at large. For some, the word diamond conjures up images of brilliant gem stones, wealth and special occasions. To the scientist, diamond is impressive because of its wide range of extreme properties. As Table 1 shows, by most measures diamond is 'the biggest and best'; it is the hardest known material, has the lowest coefficient of thermal expansion, is chemically inert and wear resistant, offers low friction, has high thermal conductivity, is electrically insulating and optically transparent from the ultra-violet (UV) to the far infrared (IR). Given these many notable properties, it should come as no surprise to learn that diamond already finds use in many diverse applications including, of course, its use as a precious gem, but also as a heat sink, as an abrasive, and as inserts and/or wear-resistant coatings for cutting tools. Obviously, given its many unique properties it is possible to envisage many other potential applications for diamond as an engineering material, but progress in implementing many such ideas has been hampered by the comparative scarcity of natural diamond. Hence the long running quest for routes to synthesise diamond in the laboratory. So called 'industrial diamond' has been synthesised commercially for over 30 years using high-pressure high-temperature (HPHT) techniques, in which diamond is crystallised from metal solvated carbon at P~50-100 kbar and T~1800-2300 K.
| Table 1: Some of the outstanding properties of diamond | |
|---|---|
| Extreme mechanical hardness (~90 GPa). | |
| Strongest known material, highest bulk modulus (1.2 x 1012 N/m2), lowest compressibility (8.3 x 10-13 m2/ N). | |
| Highest known value of thermal conductivity at room temperature (2 x 103 W / m / K). | |
| Thermal expansion coefficient at room temperature (0.8 x 10-6 K) is comparable with that of invar. | |
| Broad optical transparency from the deep UV to the far IR region of the electromagnetic spectrum. | |
| Good electrical insulator (room temperature resistivity is ~1016 Ω cm). | |
| Diamond can be doped to change its resistivity over the range 10-106 Ω cm, so becoming a semiconductor with a wide bad gap of 5.4 eV. | |
| Very resistant to chemical corrosion. | |
| Biologically compatible. | |
| Exhibits low or 'negative' electron affinity. |
World interest in diamond has been further increased by the much more recent discovery that it is possible to produce polycrystalline diamond films, or coatings, by a wide variety of chemical vapour deposition (CVD) techniques using, as process gases, nothing more exotic than a hydrocarbon gas (typically methane) in an excess of hydrogen. This CVD diamond can show mechanical, tribological, and even electronic properties comparable to those of natural diamond. There is currently much optimism that it will prove possible to scale CVD methods to the extent that they will provide an economically viable alternative to the traditional HPHT methods for producing diamond abrasives and heat sinks, whilst the possibility of coating large surface areas with a continuous film of diamond will open up whole new ranges of potential application for the CVD methods.
2 The CVD Process
Chemical vapour deposition, as its name implies, involves a gas-phase chemical reaction occurring above a solid surface, which causes deposition onto that surface. All CVD techniques for producing diamond films require a means of activating gas-phase carbon-containing precursor molecules. This generally involves thermal (e.g. hot filament) or plasma (D.C., R.F., or microwave) activation, or use of a combustion flame (oxyacetylene or plasma torches). Figure 1 illustrates two of the more popular experimental methods and gives some indication of typical operating conditions. Whilst each method differs in detail, they all share features in common. For example, growth of diamond (rather than deposition of other, less well-defined, forms of carbon) normally requires that the substrate be maintained at a temperature in the range 1000-1400 K, and that the precursor gas be diluted in an excess of hydrogen (typical CH4 mixing ratio ~1-2vol%).
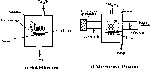 | Fig.1.Examples of two of the most common types of low pressure CVD reactor. (a) Hot filament reactor, (b) Microwave Plasma Enhanced Reactor. |
The resulting films are polycrystalline, with a morphology that is sensitive to the precise growth conditions (see later). Growth rates for the various deposition processes vary considerably, and it is usually found that higher growth rates can be achieved only at the expense of a corresponding loss of film quality. 'Quality' here is a subjective concept. It is taken to imply some measure of factors such as the ratio of sp3 (diamond) to sp2-bonded (graphite) carbon in the sample, the composition (e.g. C-C versus C-H bond content) and the crystallinity. In general, combustion methods deposit diamond at high rates (typically 100-1000 μm/hr, respectively), but often only over very small, localised areas and with poor process control leading to poor quality films. In contrast, the hot filament and plasma methods have much slower growth rates (0.1-10 μm/hr), but produce high quality films. One of the great challenges facing researchers in CVD diamond technology is to increase the growth rates to economically viable rates, (hundreds of μm/h), or even mm/hr) without compromising film quality. Progress is being made using microwave deposition reactors, since the deposition rate has been found to scale approximately linearly with applied microwave power. Currently, the typical power rating for a microwave reactor is ~5 kW, but the next generation of such reactors have power ratings up to 50-80 kW. This gives a much more realistic deposition rate for the diamond, but for a much greater cost, of course.
Thermodynamically, graphite, not diamond, is the stable form of solid carbon at ambient pressures and temperatures. The fact that diamond films can be formed by CVD techniques is inextricably linked to the presence of hydrogen atoms, which are generated as a result of the gas being 'activated', either thermally or via electron bombardment. These H atoms are believed to play a number of crucial roles in the CVD process:
- They undergo H abstraction reactions with stable gas-phase hydrocarbon molecules, producing highly reactive carbon-containing radical species. This is important, since stable hydrocarbon molecules do not react to cause diamond growth. The reactive radicals, especially methyl, CH3, can diffuse to the substrate surface and react, forming the C-C bond necessary to propagate the diamond lattice.
- H-atoms terminate the 'dangling' carbon bonds on the growing diamond surface and prevent them from cross-linking, thereby reconstructing to a graphite-like surface.
- Atomic hydrogen etches both diamond and graphite but, under typical CVD conditions, the rate of diamond growth exceeds its etch rate whilst for other forms of carbon (graphite, for example) the converse is true. This is believed to be the basis for the preferential deposition of diamond rather than graphite.
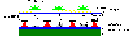
One major problem that is receiving a lot of attention is the mechanism of heteroepitaxial growth, that is, the initial stages by which diamond nucleates upon a non-diamond substrate. Several studies have shown that pre-abrasion of non-diamond substrates reduces the induction time for nucleation and increases the density of nucleation sites. Enhanced growth rates inevitably follow since formation of a continuous diamond film is essentially a process of crystallisation, proceeding via nucleation, followed by three-dimensional growth of the various microcrystallites to the point where they eventually coalesce (see Figure 2).
 |
Fig.2.Diamond initially nucleates as individual microcrystals, which then grow larger until they coalesce into a continuous film. Here, small diamond crystals are seen nucleating on a Ni surface. |
The abrasion process is usually carried out by polishing the substrate with an abrasive grit, usually diamond powder of 0.1-10 μm particle size, either mechanically or by ultrasonic agitation. Whatever the abrasion method, however, the need to damage the surface in such a poorly defined manner prior to deposition may severely inhibit the use of CVD diamond for applications in, say, the electronics industry (see later), where circuit geometries are frequently on a submicron scale. This worry has led to a search for more controllable methods of enhancing nucleation, such as ion bombardment. This is often performed in a microwave deposition reactor, by simply adding a negative bias of a few hundred volts to the substrate and allowing the ions to (i) damage the surface, (ii) implant into the lattice, and (iii) form a carbide interlayer (see later).
3 The CVD Diamond Film
The surface morphology obtained during CVD depends critically upon the gas mixing ratio and the substrate temperature. Under 'slow' growth conditions - low CH4 partial pressure, low substrate temperature - we obtain a microcrystalline film, with triangular {111} facets being most evident, along with many obvious twin boundaries (see Figure 3). {100} facets, appearing both as square and rectangular forms, begin to dominate as the relative concentration of CH4 in the precursor gas mixture, and/or the substrate temperature, is increased. Cross-sections through such microcrystalline films shows the growth to be essentially columnar (Figure 4). At still higher CH4 partial pressures the crystalline morphology disappears altogether; a film such as that shown in Figure 5 is an aggregate of diamond nanocrystals and disordered graphite.
Obviously, the crystalline morphology of a CVD diamond film is an important consideration when it comes to potential applications. A film like that shown in Figure 3 might find use as a fine abrasive coating, but most of the envisaged uses for diamond films in optics, in thermal management applications, and as possible electronic devices require that the film surfaces be as smooth as possible. One can envisage (at least) two routes to this objective: one has to either identify growth conditions which naturally result in the formation of smooth films, or to optimise ways of 'polishing' away the surface roughness of the film as grown. Both concepts are presently the subject of intense research effort.
4 The Substrate
Most of the CVD diamond films reported to date have been grown on single crystal silicon wafers, but this is by no means the only possible substrate material. What are the properties required of a substrate if it is to be suitable for supporting an adherent film of CVD diamond? One requirement is obvious. The substrate must have a melting point (at the process pressure) higher than the temperature window (1000-1400 K) required for diamond growth. This precludes the use of existing CVD techniques to diamond-coat plastics or low melting metals like aluminium. It is also helpful, though not essential, that the substrate be capable of forming a carbide. CVD of diamond on non-diamond substrates will usually involve initial formation of a carbide interfacial layer upon which the diamond then grows. Somewhat paradoxically, it is difficult to grow on materials with which carbon is 'too reactive', i.e. many of the transition metals (e.g. iron, cobalt, etc) with which carbon exhibits a high mutual solubility. Hence the appeal of substrates like Si, Mo and W - materials which form carbides, but only as a localised interfacial layer because of their modest mutual solubility with carbon under typical CVD process conditions. The carbide layer can be pictured as the 'glue' which promotes growth of the CVD diamond, and aids its adhesion by (partial) relief of stresses at the interface.
If we consider just carbon-substrate interactions, metals, alloys, and pure elements can be sub-divided into three classes exhibiting, respectively:
- Little or no C solubility or reaction. These include metals such as Cu, Sn, Pb, Ag, and Au as well as non-metals, such as Ge, sapphire, diamond itself, and graphite, although in the latter case etching will occur concurrently with diamond growth.
- C diffusion. Here, the substrate acts as a carbon sink, whereby deposited carbon dissolves into the metal surface to form a solid solution. This causes large amounts of carbon to be transported into the bulk, leading to a temporary decrease in the surface C concentration, delaying the onset of nucleation. Metals where this is significant include Pt, Pd, Rh, Fe, Ni, and Ti.
- Carbide Formation. These include metals such as Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, Fe, Co, Ni, Y, Al and certain other rare earth metals. In some metals, such as Ti, the carbide layer continues to grow during diamond deposition and can become hundreds of m thick. Such thick interfacial carbide layers may severely affect the mechanical properties, and hence the utility of CVD diamond coatings on these materials. Non-metals, such as B or Si, and Si-containing compounds such as SiO2, quartz, and Si3N4 also form carbide layers. Substrates composed of carbide themselves, such as SiC, WC, and TiC are also particularly amenable to diamond deposition.
These difficulties with growth on problematic materials have ensured the continuing popularity of silicon as a substrate material. It has a sufficiently high melting point (1683 K), it forms a localised carbide layer and it has a comparatively low thermal expansion coefficient. Tungsten and molybdenum display similar virtues and are also widely used as substrate materials. They can also be used as barrier layers - thin coatings deposited on top of certain of the problematic substrate materials mentioned above to allow subsequent diamond CVD.
5 Present Applications and Future Prospects
How is all this research effort feeding through into the marketplace? A number of areas of application are gradually beginning to appear.
Thermal management - Natural diamond has a thermal conductivity roughly four times superior to that of copper, and it is an electrical insulator: It should therefore come as little surprise to learn that CVD diamond is now being marketed as a heat sink for laser diodes and for small microwave integrated circuits. The natural extrapolation of this use in circuit fabrication ought to be higher speed operation, since active devices mounted on diamond can be packed more tightly without overheating. Reliability can be expected to improve also since, for a given device, junction temperatures will be lower when mounted on diamond.
Cutting tools - CVD diamond is also finding applications as an abrasive and as a coating on cutting tool inserts. CVD diamond-coated drill bits, reamers, countersinks etc. are now commercially available for machining non-ferrous metals, plastics, and composite materials. Initial tests indicate that such CVD diamond-coated tools have a longer life, cut faster and provide a better finish than conventional tungsten carbide tool bits.
Wear Resistant Coatings - In both the previous applications, CVD diamond is performing a task that could have been fulfilled equally well by natural diamond if economics were not a consideration. However, there are many other applications at, or very close to, the market-place where CVD diamond offers wholly new opportunities. Wear resistant coatings are one such use. The ability to protect mechanical parts with an ultra-hard coating, in for example, gearboxes, engines, and transmissions, may allow greatly increased lifetimes of components with reduced lubrication.
The phrase 'non-ferrous' is worth emphasising here since it reminds us of one of the biggest outstanding challenges in the application of diamond film technology - whether as a wear-resistant coating or as a fine abrasive. In any application where friction is important the diamond-coated tool bit will heat up and, in the case of ferrous materials (be it the tool substrate or the workpiece) the diamond coating will ultimately react with the iron and dissolve. Intense research efforts into suitable barrier layer materials to allow diamond coating of iron and steel machine parts are currently underway.
Optics - Because of its optical properties, diamond is beginning to find uses in optical components, particularly as protective coatings for infrared (IR) optics in harsh environments. Most IR windows currently in use are made from materials such as ZnS, ZnSe, and Ge, which, whilst having excellent IR transmission characteristics, suffer the disadvantage of being brittle and easily damaged. A thin protective barrier of CVD diamond may provide the answer, although it is more likely that future IR windows will be made from free-standing diamond films grown to a thickness of a few mm using improved high growth-rate techniques. However, a major consideration when using polycrystalline CVD diamond films for optics is the flatness of the surface, since roughness causes attenuation and scattering of the transmitted IR signal, with subsequent loss of image resolution. Hence the current interest in techniques for smoothing diamond films we mentioned very briefly earlier.
Electronic devices - the possibility of doping diamond and so changing it from being an insulator into a semiconductor opens up a whole range of potential electronic applications. However, there are a number of major problems that need to be overcome if diamond-based electronic circuits are to be achieved. Principal among these is the fact that CVD diamond films are polycrystalline and hence contain grain boundaries, twins, stacking faults, and other defects, which all reduce the lifetime and mobilities of carriers. Active devices have been demonstrated using homoepitaxially-grown diamond on natural or synthetic diamond substrates but, to date, there have been no corroborated reports of heteroepitaxial growth of device-quality diamond on non-diamond substrates. This remains a major limiting factor in the development of diamond devices. Nevertheless, the effect of grain boundaries and defects upon electronic carriers in the very best polycrystalline diamond films remains to be ascertained and, clearly, this possible route to active diamond devices cannot yet be ruled out.
Another outstanding problem hindering potential diamond electronics is the difficulty in producing n-type doping. P-type doping is relatively straightforward, since addition of a few percent of B2H6 to the CVD process gas mixture is all that is required to incorporate B into the lattice. However, the close packing and rigidity of the diamond lattice makes doping with atoms larger than C very difficult. This means that the dopants which are routinely used to n-dope Si, such as P or As, cannot easily be used for diamond, and so alternative dopants, such as Li are being investigated.
One further difficulty that must be overcome if diamond devices are to be realised is the ability to pattern the diamond films into the required micron or even submicron geometries. Dry etching using O2-based plasmas can be used, but etch rates are slow and the masking procedure complex. Alternative patterning methods include laser ablation, or selective nucleation. There are many variants of this latter process, but all involve trying to mask off certain areas of the substrate so allowing diamond to grow only in selected regions. A typical process scheme involves coating a Si substrate with a thin layer of SiO2 which is then patterned using standard photolithographic and dry etching methods to expose areas of Si. The wafer is then ultrasonically abraded, and the oxide layer is then stripped in HF. CVD diamond is then grown, nucleating preferentially on the abraded Si areas rather than the unabraded areas that were beneath the mask. Examples of patterned diamond films produced in this way are are given in Figs. 6a, 6b and 6c.
 | Fig.6a |
 | Fig.6b |
 | Fig.6c |
Patterned diamond films produced by selective area deposition, showing that resolutions of a few microns are possible.
Despite these difficulties, CVD diamond-based devices are gradually beginning to appear, albeit with imperfect characteristics. Piezoelectric effect devices, radiation detectors, and even the first field effect transistors have all been reported recently, with the likelihood that some of the simpler devices will become commercially available in the near future. One potential application which is causing a great deal of interest at the moment is the idea of using diamond as an electron emitter in flat panel displays (see Figure 7).
The electronic properties of diamond are such that when it is biased negatively in vacuum, electrons are ejected from its surface. This process is also common to most metals, except that in metals the electron has to overcome an energy barrier, or work function, to escape from the surface. In diamond this barrier has been measured and found to be very small, maybe even negative, and this has given rise to the term 'negative electron affinity'. In practice, this means that devices based upon diamond's electron emission properties could consume very low power levels and hence be extremely efficient. The electrons emitted from the surface are accelerated using a positively biased grid, and then strike a target. The target material determines what type of device is made. If the target is a phosphor screen, light will be emitted where the electrons strike it, and we have the basis for a flat panel display. Each emitting diamond crystal, or group of crystals would form a 'pixel' on the screen. Unlike their major competitors (liquid crystal displays), diamond cold cathode emission displays would have high brightness, have a large viewing angle, and most importantly, have the ability to be scaled up to large sizes (maybe even metres square!). Alternatively, if the target material is a conductor capable of collecting current, the emission device could form the basis of an ultra-fast switch, so suggesting the possibility of faster computers.
Composite Materials - Another interesting new development in diamond technology is the ability to deposit CVD diamond onto the outer surfaces of metal wires or non-metallic fibres (see Figure 8). Such diamond-coated fibres have measured modulus values close to that expected for diamond, making them extremely stiff for their weight. If growth rates can be increased to economically viable levels, such diamond fibres may find uses as reinforcing agents in metal matrix composites, allowing stronger, stiffer and lighter load-bearing structures to be manufactured for use in, say, aerospace applications. Furthermore, etching out the metal core of the diamond-coated wire using a suitable chemical reagent yields free-standing diamond tubes, or hollow diamond fibres. These too have potential applications for reinforcing smart composites, since the hollow cores may provide conduits for sealant, coolant, or sensors to be placed into the reinforced structure. Two-dimensional mattings or weaves of diamond-coated fibres have also been suggested as reinforcing agents.
 |
Fig.8 A diamond-coated tungsten wire. The metal core is 125 μm in diameter, with a coating thickness of about 30μm of diamond. |
6 Summary
Most of the scientific research effort into CVD diamond technology has been concentrated within the past five years yet, already, some of the more obvious applications, such as cutting tools and heat sinks, have reached the market-place. With the current rapid rate of progress, it should not be too long before this fledgling technology begins to make a significant impact in many areas of modern life. However, several issues need to be addressed before this can happen. Growth rates need to be increased (by one or more orders of magnitude) without loss of film quality. Deposition temperatures need to be reduced by several hundred degrees, allowing low melting point materials to be coated and to increase the number of substrates onto which adherent diamond films can be deposited. A better understanding of the nucleation process is required, hopefully leading to an elimination of the poorly controlled pre-abrasion step. Substrate areas need to be scaled up, again without loss of uniformity or film quality. For electronic applications, single crystal diamond films are desperately needed, along with reliable techniques for patterning and controlled n- and p-type doping.
At present, there is a huge amount of work being done throughout the world on solving these issues, and progress is being made seemingly on a daily basis. If this continues, the future for CVD diamond looks bright indeed.
7 Acknowledgements
Financial support from the Royal Society, the EPSRC and the Department of Trade and Industry is gratefully acknowledged, as is the help, advice, and encouragement offered by the many other members of the University of Bristol 'Diamond Group'.
8 Further Reading
A more detailed review of the subject of CVD diamond, including many of the source references can be found in: M.N.R. Ashfold, P.W. May, C.A. Rego and N.M. Everitt, Chem. Soc. Rev., 23 (1994) 21, and in Synthetic Diamond: Emerging CVD Science and Technology, Edited by K.E. Spear and J.P. Dismukes (Wiley, 1994), and in P.W. May, "Diamond Thin Films: A 21st Century Material" Phil. Trans. R. Soc. Lond. A, 358 (2000) 473-495.