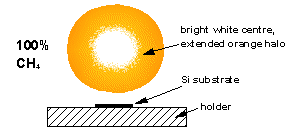
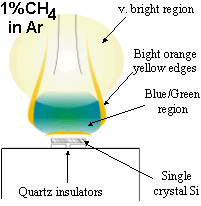
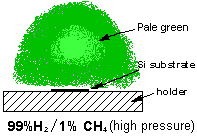
Microwave Plasma Enhanced Chemical Vapour Deposition of Diamond
Microwave Plasma Enhanced Chemical Vapour Deposition of Diamond
Microwave plasma enhanced chemical vapour deposition (MWPECVD) is used at Bristol to deposit CVD diamond under varying growth conditions. Films can then be analysed ex situ by Scanning Electron Microscopy (SEM), Laser Raman Spectroscopy (LRS), Field Emission studies, AES, SIMS etc. Previously we did a lot of work studying the gas-phase chemistry of these plasmas using various laser spectroscopic methods and an in situ molecular-beam mass spectrometer. Nowadays our two MWPECVD systems are used for growth experiments and to make devices such as betavoltaic or gammavoltaic devices.
Plasma-assisted deposition methods
Plasma-assisted deposition techniques are very popular methods for growing CVD diamond. Plasma systems offer uniform films over larger substrate areas than hot filament CVD growth, with the possibilty of industrial scale up by using more powerful reactors. The plasma in a microwave system is detached from any reactor surface, hence no impurities from reactor construction materials enter the film bulk during deposition. In hot-filament CVD, incorporation of filament materials into the film occurs during deposition and this (combined with limited deposition area) make hot-filament methods useless for high-purity commercial applications of diamond. However hot-filament reactors are cheaper and simpler than microwave reactors and a lot of useful research is done in them.
Our Microwave systems
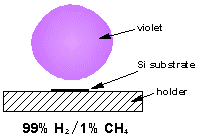
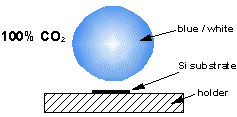
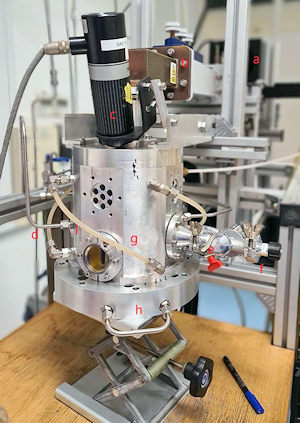
Both of our MW systems are top-hat (ASTeX-style) reactors consisting of a microwave generator coupled to the top of a cylindrical, water cooled, stainless steel chamber. A resonant mode, created by the microwaves, is supported in the chamber and the reactant gases are heated and excited to form a plasma ball. The substrate sits ~1 mm below the visible edge of the plasma ball, on top of molybdenum substrate holder. The reactors are also equipped with diagnostic ports.
 |
 |
| The 2 kW MW reactor used for undoped diamond. | The 3 kW MW reactor used for B-doped diamond. |
We have 2 reactors, one for boron-doping and one for non-boron work. The boron-reactor has a 3 kW MW power supply and has the usual CVD gases attached to it (CH4, H2, O2, Ar, etc.), plus also 1%B2H6 in H2 for doping. The non-boron reactor has a 2 kW power supply and has the same gases (except B2H6), plus many others for use in plasma experiments, such as N2, NH3, etc.
Both reactors are capable of growing high-quality polycrystalline diamond films at rates of around 10 μm h-1 uniformly over a substrate area of 2×2 cm. Films of many 10's of μm can easily be grown in a few hours, or even 100's of μm by combining together several day's worth of growth runs. The reactors can also grow single-crystal epitaxial layers (undoped or B-doped) onto SCD substrates. This is useful for adding a conducting epitaxial layer to a commercial SCD substrate which is necessary to prevent charging during XPS analysis.
The Plasma Balls
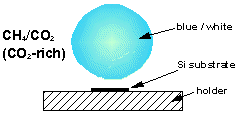
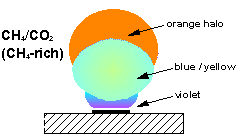
Over the years we have studied plasmas made from many different types of gas mixture. Photos and diagrams of these various plasma balls can be seen below.
 Low Temperature Diamond CVD Using CO2/CH4 Gas Mixtures
Low Temperature Diamond CVD Using CO2/CH4 Gas Mixtures
The now well established conditions for diamond growth include the use of substrate temperatures >>700°C and a carbon-containing precursor gas diluted in excess hydrogen (typically <5% CH4 in H2). A major goal in the field of diamond CVD is the lowering of substrate temperatures required for growth, as this could permit the use of a much wider range of substrate materials of industrial importance, such as aluminum, GaAs, nickel and steel.
Many gas mixtures containing varying ratios of O, C and H have been investigated in the search for a viable low temperature diamond deposition process. Work within our group has centred on microwave Plasma CVD diamond deposition using CO2/CH4 gas mixtures. Investigations of both low-temperature growth and gas-phase plasma chemistry have been carried out, results of which are discussed below.
Low Temperature Diamond Growth
It has been found that the highest quality diamond is obtained using the mixture 50%CH4/50%CO2. This gas mixture has also been found to allow diamond growth at substrate temperatures <500°C although a clear reduction in the crystallinity of the films with reduced temperature can be seen.
Lowering substrate temperature also has the effect of lowering film growth rate . Using these growth rate data, an Arrhenius plot can be produced which yields an overall activation energy for diamond deposition of 28 kJ mol-1. This is lower than previously obtained for CH4/H2 systems and hints at a different surface chemistry in the two systems.
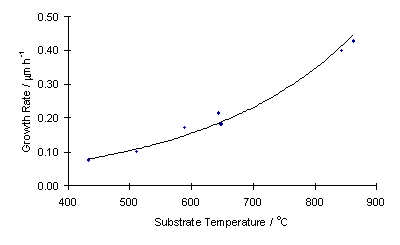 |
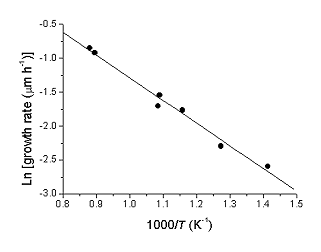 |
| Growth rate vs substrate temperature | Arrhenius plot of growth rate data |
CO2/CH4 plasma chemistry
Studies have shown that CO2/CH4 mixtures containing >50% CO2 result in no film deposition, 50%CO2/50%CH4 gives optimum diamond growth whilst increasing %CH4 above 50% produces increasingly graphitic material. In order to explain these observations molecular-beam mass spectrometry was used to make measurements of plasma species concentrations over a wide range of mixing ratios (0-80% CH4). Computer simulation of these results was then carried out using the CHEMKIN II computer package thus allowing the important plasma reactions to be identified.
- CO2 + CH4 → 2CO + 2H2
- CO2 + H2 → CO + H2O
- H2O + C2H2 → CH4 + CO
Reaction 3 is important because it leads to a peak in CH4 (and therefore CH3) concentration at 50% input CH4. CH3 is believed to be the growth species in diamond CVD using CH4/H2 gas mixtures. The peak in CH3 concentrations at the same composition as the optimum diamond growth suggests that CH3 is also the diamond growth species for CO2/CH4 gas mixtures.
The different activation energy obtained for 50%CO2/50%CH4 compared with CH4/H2 gas mixtures suggests a difference in the growth mechanism. This could be due to a difference in the surface termination of the growing diamond surface which is thought to be performed by H in CH4/H2 systems. Given the relatively high concentration of CO present at 50%CO2/50%CH4 it seems reasonable that at least some of the diamond surface could be terminated with CO (or HCO) in this system.
Related Papers
- S.M. Leeds, P.W. May, E. Bartlett, M.N.R. Ashfold, and K.N. Rosser, "Molecular Beam Mass Spectrometry studies of the gas phase chemistry occurring during Microwave Plasma assisted Chemical Vapour Deposition of Diamond", Diamond Relat. Mater., 8 (1999) 1377-82
- S.M. Leeds, P.W. May, M.N.R. Ashfold and K.N. Rosser, "Molecular Beam Mass Spectrometry studies of Nitrogen additions to the gas phase during Microwave Plasma Assisted Chemical Vapour Deposition of Diamond", Diamond Relat. Mater., 8 (1999) 226-230
- A.J. Eccles, T.A. Steele, A. Afzal, C.A. Rego, W. Ahmed, P.W. May and S.M. Leeds, "Influence of B- and N-doping Levels on the Quality and Morphology of CVD Diamond" Thin Solid Films, 343-344 (1999) 627-631.
- M.A. Elliott, P.W. May, J. Petherbridge, S.M. Leeds and M.N.R. Ashfold, "Optical Emission Spectroscopic Studies of Microwave Enhanced Diamond CVD using CH/CO2 Plasmas" Diamond Relat. Mater., 9 (2000) 311-316
- J.R. Petherbridge, P.W. May, S.R.J. Pearce, K.N. Rosser and M.N.R. Ashfold, "Low temperature diamond growth using CO2/CH4 plasmas: molecular beam mass spectrometry and computer simulation investigations" J. Appl. Phys., 89 (2001) 1484-1492.
- J. Petherbridge, P.W. May, S.R.J. Pearce, K.N. Rosser and M.N.R. Ashfold, "Molecular beam mass spectrometry investigations of low temperature diamond growth using CO2/CH4 plasmas", Diamond Relat. Mater., 10 (2001) 393-398.
- J.R. Petherbridge, P.W. May, G.M. Fuge, G.F. Robertson, K.N. Rosser and M.N.R. Ashfold, "Sulfur doping of diamond films: spectroscopic, electronic and gas-phase studies", J. Appl. Phys. 91 (2002) 3605-3613.
- J.R. Petherbridge, P.W. May, G.M. Fuge, K.N. Rosser and M.N.R. Ashfold, "In situ plasma diagnostics of the chemistry behind sulfur doping of CVD diamond films", Diamond Relat. Mater. 11 (2002) 301-306
- J.R. Petherbridge, P.W. May, E.J. Crichton, K.N. Rosser and M.N.R. Ashfold, "Sulphur addition to microwave activated CH4/CO2 gas mixtures used for diamond CVD: growth studies and gas phase investigations", Phys. Chem. Chem. Phys. 4 (2002) 5199-5206.
- J.R. Petherbridge, P.W. May, M. Baines, D. Cherns, "Observations of nanotube and 'celery' structures following diamond CVD on single crystal diamond substrates", Diamond Relat. Mater. 12 (2003) 1858-61
- A. Cheesman, J. A. Smith, M. N. R. Ashfold, N. Langford, S. Wright, G. Duxbury, "Application of a Quantum Cascade Laser for Time-Resolved, in Situ Probing of CH4/H2 and C2H2/H2 Gas Mixtures during Microwave Plasma Enhanced Chemical Vapor Deposition of Diamond", J. Phys. Chem. A 110 (2006) 2821-8.
- Yu.A. Mankelevich, P.W. May, "New insights into the mechanism of CVD diamond growth: Single Crystal diamond in MW PECVD Reactors", Diamond Relat. Mater. 17 (2008) 1021-28
- P.W. May, Yu. A. Mankelevich, "From ultrananocrystalline diamond to single crystal diamond growth in hot filament and microwave plasma-enhanced CVD reactors: A unified model for growth rates and grain sizes" J. Phys. Chem. C 112 (2008) 12432–12441. [doi: 10.1021/jp803735a]
- J. Ma, J.C. Richley, M.N.R. Ashfold, Yu.A. Mankelevich, "Probing the plasma chemistry in a microwave reactor used for diamond chemical vapor deposition by cavity ring down spectroscopy", J. Appl. Phys. 104 (2008) 103305.
- Yu.A. Mankelevich, M.N.R. Ashfold, J. Ma, "Plasma-chemical processes in microwave plasma-enhanced chemical vapor deposition reactors operating with C/H/Ar gas mixtures" J. Appl. Phys. 104 (2008) 113304. [doi: 10.1063/1.3035850]
- J. Ma, M. N. R. Ashfold, Yu. A. Mankelevich, "Validating optical emission spectroscopy as a diagnostic of microwave activated CH4/Ar/H2 plasmas used for diamond chemical vapor deposition", J. Appl. Phys. 105 (2009) 043302, 1-12. [doi: 10.1063/1.3078032]
- O.J.L. Fox, J. Ma, P.W. May, M.N.R. Ashfold, Yu.A. Mankelevich, "The role of inert gas in MW-enhanced plasmas for the deposition of nanocrystalline diamond thin films", Diamond Relat. Mater. 18 (2009) 750-58 [doi: 10.1016/j.diamond.2009.01.004]
- J. Ma, A. Cheesman, M.N.R. Ashfold, K.G. Hay, S. Wright, N. Langford, G. Duxbury, Yu.A. Mankelevich, "Quantum cascade laser investigations of CH4 and C2H2 interconversion in hydrocarbon/H2 gas mixtures during microwave plasma enhanced chemical vapor deposition of diamond", J. Appl. Phys. 106 (2009) 033305. [doi: 10.1063/1.3176971]
- Jie Ma, J.C. Richley, D.R.W. Davies, A. Cheesman, M.N.R. Ashfold and Y.A. Mankelevich, "Spectroscopic and modelling investigations of the gas phase chemistry and consumption in microwave plasma activated B2H6/Ar/H2 gas mixtures", J. Phys. Chem. A 114 (2010) 2447-63. [doi: 10.1021/jp9094694]
- Jie Ma, J.C. Richley, D.R.W. Davies, M.N.R. Ashfold and Y.A. Mankelevich, "Spectroscopic and modeling investigations of the gas phase chemistry and composition in microwave plasma activated B2H6/CH4/Ar/H2 gas mixtures", J. Phys. Chem. A 114 (2010) 10076-89. [doi: 10.1021/jp104532y]
- J.C. Richley, O.J.L. Fox, M.N.R. Ashfold and Yu.A. Mankelevich, "Combined experimental and modeling studies of microwave activated CH4/H2/Ar plasmas for microcrystalline, nanocrystalline, and ultrananocrystalline diamond deposition", J. Appl. Phys. 109 (2011) 063307. [doi: 10.1063/1.3562185]
- M.W. Kelly, J.C. Richley, C.M. Western, M.N.R. Ashfold, Y.A. Mankelevich, "Exploring the Plasma Chemistry in Microwave Chemical Vapor Deposition of Diamond from C/H/O Gas Mixtures", J. Phys. Chem. A 116 (2012) 9431-46. [doi: 10.1021/jp306190n
- J.C. Richley, M.W. Kelly, M.N.R. Ashfold, Y.A. Mankelevich, "Optical Emission from Microwave Activated C/H/O Gas Mixtures for Diamond Chemical Vapor Deposition", J. Phys. Chem. A 116 (2012) 9447-58. [doi: 10.1021/jp306191y]
- B.S. Truscott, M.W. Kelly, K.J. Potter, M. Johnson, M.N.R. Ashfold, "Microwave Plasma-Activated Chemical Vapour Deposition of Nitrogen-Doped Diamond, I: N2/H2 and NH3/H2 Plasmas", J. Phys. Chem. A 119 (2015) 12962-12976. [doi: 10.1021/acs.jpca.5b09077].
- B.S. Truscott, M.W. Kelly, K.J. Potter, M.N.R. Ashfold, and Yu.A. Mankelevich, "Microwave Plasma-Activated Chemical Vapour Deposition of Nitrogen-Doped Diamond, II: CH4/N2/H2 Plasmas" J. Phys. Chem. A 120 (2016) 8537-8549. pdf [doi: 10.1021/acs.jpca.6b09009].
- M.N.R. Ashfold, E.J.D. Mahoney, S. Mushtaq, B S. Truscott and Yu.A. Mankelevich, "What [plasma used for growing] diamond can shine like flame?", Chem. Comm. 53 (2017) 10482. [doi: 10.1039/c7cc05568d].
- E.J.D. Mahoney, B.S. Truscott, M.N.R. Ashfold, Yu.A. Mankelevich, "Optical Emission from C2- Anions in Microwave-Activated CH4/H2 Plasmas for Chemical Vapor Deposition of Diamond", J. Phys. Chem. A 121 (2017) 2760-72. [doi: 10.1021/acs.jpca.7b00814].
- A. Croot, E.J.D. Mahoney, H. Dominguez-Andrade, M.N.R. Ashfold, N.A. Fox, "Diamond chemical vapor deposition using a zero-total gas flow environment", Diamond Relat. Mater. 109 (2020) 108011. [doi: 10.1016/j.diamond.2020.108011].
- M.N.R. Ashfold, Yu.A. Mankelevich, "Two-dimensional modeling of diamond growth by microwave plasma activated chemical vapor deposition: Effects of pressure, absorbed power and the beneficial role of nitrogen on diamond growth", Diamond Relat. Mater. 137, (2023) 110097. pdf [doi: 10.1016/j.diamond.2023.110097]