Introduction
| Title Page | ||||||
| Introduction | ||||||
| History of PVC | ||||||
Manufacture
of PVC
|
||||||
| Disposing of PVC | ||||||
| References |
|
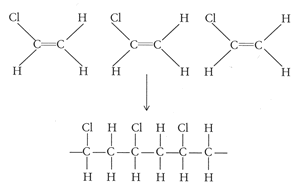
Polyvinylchloride (PVC) [-(-CH2 -CHCl-)n-] is one of the three most important polymers currently used worldwide. This is because PVC is one of the cheapest polymers to make and has a large range of properties so can be used to make hundreds of products. PVC is formed by the polymerisation of vinyl chloride (chloroethane) monomer units:
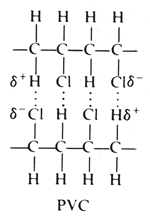
PVC consists of polar molecules which are attracted to each other by dipole-dipole interactions due to electrostatic attractions of a chlorine atom in one molecule to a hydrogen atom in another atom:
These considerable intermolecular attractions between polymer chains make PVC a fairly strong material. Uncompounded PVC is colourless and rigid and possesses poor stability towards heat and light. However, the use of additives/stabilisers enables us to change the properties of the PVC to how we desire. |