


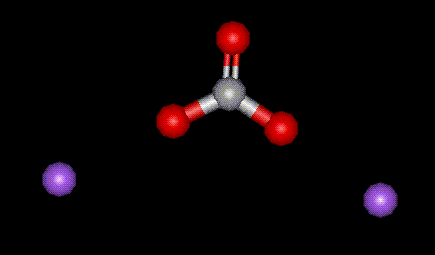
 Sodium carbonate is naturally occurring in alkaline waters, however it is also synthesised by the Solvay process or by electrolysis of sea water. Sodium carbonate is used as an acidity regulator, particularly in beer making. Excessive ingestion may result in stomach upset.
Sodium carbonate is naturally occurring in alkaline waters, however it is also synthesised by the Solvay process or by electrolysis of sea water. Sodium carbonate is used as an acidity regulator, particularly in beer making. Excessive ingestion may result in stomach upset.| Other names: | sodium carbonate, soda ash, disodium carbonate |
| Molecular formula: | Na2 CO3 |
| CAS No: | 497-19-8 |
| Physical appearance: | white odourless powder |
| Melting point: | 851 C |
| Other information: | May irritate the eyes and respiratory tract. |
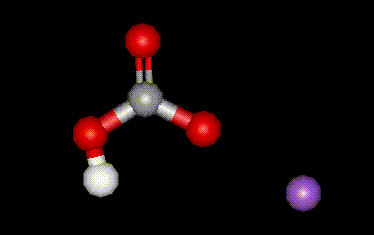 Sodium hydrogen carbonate is used in food mainly as a raising agent, but also as a base. It is prepared synthetically.
Sodium hydrogen carbonate is used in food mainly as a raising agent, but also as a base. It is prepared synthetically.| Other names: | bicarbonate of soda, baking soda, sodium hydrogen carbonate |
| Molecular formula: | NaHCO3 |
| CAS No: | 144-55-8 |
| Physical appearance: | white powder or crystals |
| Melting point: | 50 C |
| Other information: | May irritate the eyes |