|
Buckminsterfullerene The other allotrope of carbon is buckminsterfullerene, named after the architect and inventor Richard Buckminster Fuller who created the geodesic domes (figure 4),
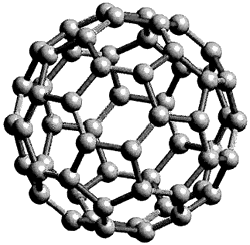
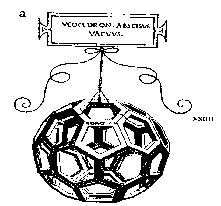
which inspired the realisation of the structure of C60 (figure 5). The structure of a regular truncated icosahedron was already known by Leonardo de Vinci in the 1500’s (figure 6).
Another interpretation form the same period was that by Albrecht Dürer (figure 7). In the twentieth century theoretical solutions concerning molecular group symmetry preceded the discovery of C60 in the laboratory by several decades. Tisza, in 1933, considered the molecular group symmetry and point group symmetry for icosahedral molecules and in 1970 Osawa proposed that an icosahedral form of C60 could be chemically stable. In 1987 Harry Kroto wrote an article in Nature, a scientific journal, which explained the discovery of C60, a truncated icosahedron. Fullerenes are a new family of non-equilateral compounds of carbon discovered by researchers at the University of Sussex and the University of Rice in the United States. There structures are composed of hexagons and pentagons a bit like in a football; they all have closed structures with the carbon in cages. Each carbon has a bond between three others and its hybridisation is sp2 (in contrast the hybridisation in diamond is sp3). Buckminsterfullerene is neither aromatic nor super aromatic and its delocalisation of electron density is poor. C60 behaves like an alkene which has insufficient electrons so it reacts with electron rich species. The discovery of the buckyball was collaboration between the universities of Rice and Sussex. A laser vaporisation technique was used to produce the target of clusters of atoms. Kroto realised that is they used a target of graphite, the clusters arranged themselves perfectly for the formation of chains of carbon. In September 1985 the technique sounded a plasma product by the vaporisation of lasers by mass spectrometry. Experiments confirmed that clusters or chains of carbon were formed. During the experiments it was noted that the coordination for C60 showed great stability. The configuration of pentagons in the structure lends stability. There were five examples presented in Nature to how and confirm the stability of the molecule. Firstly: the valence of carbon is consistent with the normal requirements for carbon, so each atom is bonded to three others by two single bonds and one double bond. Secondly: the measure of resonance proves that the molecule is stable, in C60 there are 12,500 resonance structures. Hydrocarbons with five or six membered rings are abundant and stable. Thirdly: after the research of W. Barth and R. Lawton it is clear that a structure in which a pentagon is completely surrounded by hexagons is stable. Fourthly: geodesic structures prefer symmetric isomers so the tension can be equally distributed over all the atoms. In structures where the curvature is localised the most vulnerable position for attack is at the site of tension. Finally: closed electronic structures are preferred. C60 is the smallest closed fullerene with each pentagon separated without tension. Buckminsterfullerene therefore dominated the chemistry of fullerenes. Professors Curl and Smalley of the United States and Professor Sir Kroto from the United Kingdom were awarded the Nobel Prize in chemistry in 1996 for the discovery of the buckyball.
Home ||
Diamond ||
Graphite ||
Buckyballs ||
Nanotubes ||
Fullerenes
Samantha Shanley, School of Chemistry, University of Bristol |