|
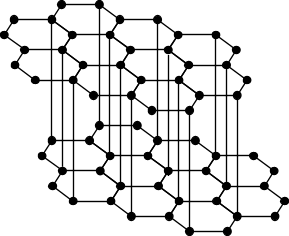
Graphite As surprising as it seems, graphite is the most stable of the allotropes. Graphite is black and soft (figure 3).
Figure 3. Graphite Due to the difference in layers of graphite, the pi orbitals enable it to conduct electricity (the conductivity of diamond is 10-18 that of the levels of graphite). Graphite exists in two forms: that of a hexagon and a rhombus. The layers of hexagons are arranged in parallel and are linked by Van der Waals forces. The separation between the layers is 3.35 x 10-10 metres, about the sum of the layers of Van der Waals for carbon. This indicates that the forces between the layers are not strong. Actually the layers slide over each other so graphite is a lubricant. The difference between the structures of graphite is caused by the arrangement of hexagon in the forms of chairs and boats. Nevertheless each atom has a coordination number of three so the atoms are arranged trigonally. The bonds between the carbons are multiple. The structure of graphite permits the penetration of molecules and ions between layers to form intercalculation compounds, or lamellars. These lamellars form spontaneously when the reagents are close to each other.
Home ||
Diamond ||
Graphite ||
Buckyballs ||
Nanotubes ||
Fullerenes
Samantha Shanley, School of Chemistry, University of Bristol |