| Julian
Eastoe Research Group
Caroline Seguin |
|||||||||
|
Last updated 25-01-2006
|
Caroline joined the group from Montpellier in the sunny south of France where she was studying condensed matter and properties of metals. Sponsored by Huntsman, she is now investigating surfactants in mixtures of water and non-aqueous polar solvents, using De Noüy tensiometry, polarising light microscopy and SANS (Small Angle Neutron Scattering).
It
is well known that surfactant molecules self-assemble in water to form
micelles, vesicles, or a variety of liquid crystalline phases.1 Surfactants also aggregate in non-aqueous solvents, but interestingly micelle formation in non-aqueous solvents has attracted little attention as compared to the extensive studies of aqueous surfactant solutions.2 To differentiate from ‘inverted’ micelles which are formed in non-polar organic solvents, the term ‘solvophobic interaction’ has been introduced by various researchers, in analogy with ‘hydrophobic interaction’ causing micellisation in aqueous media. Micelles formed due to ‘solvophobic interactions’ in polar non-aqueous solvents are similar in many respects to those in aqueous media.
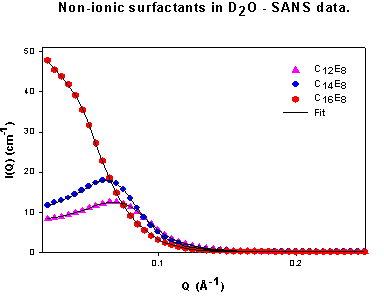
The aim of the project is to study the behavior of non-ionic surfactants in pure non-aqueous solvents, and mixtures of these solvents with water. Work is carried out at high concentration in order to observe adsorption and aggregation behavior. Certain ionic and zwitterionic surfactants are also assessed. Surface tension (surface pressure) is determined using the De Noüy ring tensiometry while Small-angle neutron scattering (SANS) is used to obtain information on the shape, radii and volume fraction of the micelles.
References. [1]
Martino, A.; Kaler, E. W. Colloids Surf. A 1995, 91-99. [2] Akhter, M. S. Colloids Surfaces A. 1999, 150, 25-30. |
 Surfactants
in non-aqueous media
Surfactants
in non-aqueous media