|
Prof.
Julian Eastoe
|
Photo-surfactants
If a surfactant molecule contains a
suitable chromophore, thereby giving it isomer-dependent properties,
illumination can be used to affect adsorption and aggregation.
We have developed highly optimised photo-surfactants, and some
exciting new results are shown below.
These may be considered as the next generation of surfactants,
with added functionality and tuneability.
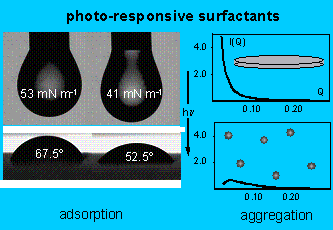
Photo-active
systems revolutionise surfactants from mundane everyday molecules, into
sophisticated additives, which can perform key functions in various
areas, for example targeted delivery of drugs and carrier liquids,
printing and surface coating.
CO2-active
surfactants
Owing to
its accessible critical point (31.1 °C and 73.8 bar) CO2 has
significant environmental and economic advantages over currently used
solvents. However, weak
intermolecular forces in CO2 result in very low solubility of
polars. Formation of a
water-in-CO2 (w/c) microemulsion or emulsion is one way of
overcoming the problem, but this necessitates developing specialised CO2-compatible
surfactants. Fluorocarbons
are very effective, but impractical for real applications
owing to their high cost. We
have designed more economic hydrocarbons (A and B below) to be CO2-active;
these are analogues of the well-known AOT, which is insoluble.
The terminal t-butyl
groups give the surfactants sufficiently low surface energies, making
them CO2-soluble. This
finding opens the door to new cost-effective surfactants for these
applications.
Polymerisable surfactants
A central theme in materials
chemistry is template-directed fabrication of nano-structured
architectures. We have
developed new self-assembled organic phases, made from reactive
surfactant monomers (surfmers). These
compounds bear polymerisable chains and/or counterions which can be used
to “zip up” aggregates without destroying their initial
nanostructure, providing optimised reaction templates.
Shown below are cylindrical nano-particles of BaSO4,
which have been grown in encapsulated rod-shaped microemulsion droplets,
stabilised by a partially polymerised monolayer.
The ideas are quite general and can be applied in any region of
the surfactant phase diagram.
|