|
Home
The molecule Physical properties Chemical properties Antimicrobial properties Structure-activity relationships Biosynthesis Chemical syntheses References |
Chemical properties
The reactions that tetracyclines undergo are generally of a sophisticated nature, dictated by the complex functionality and the sensitivity of the molecules to mild reaction conditions (acid, base, heat) [8].
Acidic conditions
When exposed to dilute acid conditions, tetracycline undergoes dehydration to yield anhydrotetracycline. Anhydroterramycin suffers further cleavage and lactonization to apoterramycin:
.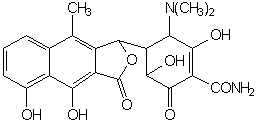
Diluted acid promotes epimerization at C-4 as well.Basic conditions
Mild alkali attacks 11a carbon of tetracycline, which is transformed to isotetracycline:
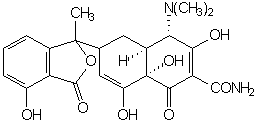
Formation of complexes
Tetracycline possesses a great tendency to form complexes with a number of chemical species, due to its B- and C-ring oxygen atoms:

It complexes most readily with Fe3+, Fe2+, Cu2+, Ni2+, Co2+, Zn2+, Mn2+, Mg2+, Ca2+, Be2+, Al3+ among metal ions, phosphates, citrates, salicylates, p-hydroxybenzoates, saccharin anion, caffiene, urea, thiourea, polivinylpyrrolidone, serum albumin, lipoproteins, globulins, and RNA [11].