 CVD Gas-Phase Analysis using In Situ Molecular-Beam Mass Spectrometry
CVD Gas-Phase Analysis using In Situ Molecular-Beam Mass Spectrometry
Some of the earliest diagnostic work we did in the mid 1990s involved studying the gas-phase chemistry occuring during diamond CVD using an in situ Quadrupole Mass Spectrometer (QMS). The work provided a wealth of informaton about the species present near the hot filament or in the plasma, and proved essential when were developing the model for diamond growth and subsequent Monte Carlo simulations. However, work ended on this project in 2002.
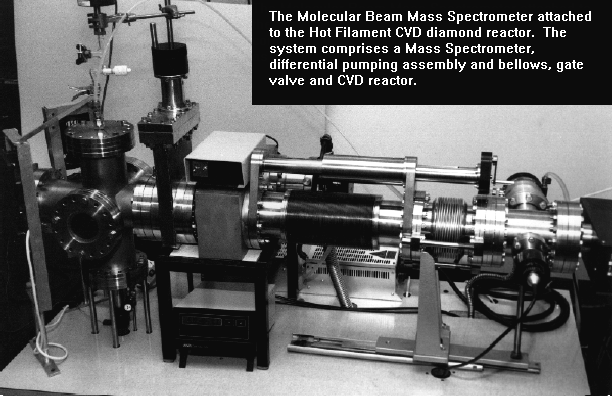 The original MBMS system on the right attached to a hot-filament CVD reactor. |
System descriptionThe mass spectrometer sampled from a continuous molecular beam extracted from a CVD chamber through a viewpoet flange. This work was begun by PhD student Roland Tsang who used MBMS to study the gas phase environment during hot filament assisted CVD. He was followed by another PhD student, Stuart Leeds, who modified the sampling cone assembly to enable us to apply the same techniques to microwave plasma CVD reactors. Later, PhD student James Petherbridge used the system to study low-temperature growth using CO2/CH4 gas mixtures, as well as H2S additions for sulfur doping experiments. Finally, the system was modified by PhD students Ed Crichton and Oliver Fox to allow investigation of the high-pressure Ar/CH4/H2 gas mixtures used to deposit UNCD films. This involved a complete rebuild of the reactor, with the MS now sampling from below through a small orifice in the substrate holder. |

Schematic diagram of the MBMS attached to a MW reactor.
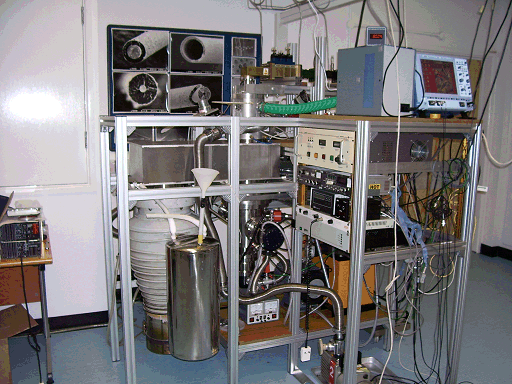 The final system consisted of a Microwave plasma reactor (right) to which the molecular-beam sampling apparatus was attached. A 100 μm-diameter orifice in the substrate holder (rather than via the viewport) was used to extract a sample of the plasma gas as a supersonic gas expansion. The region immediately behind the orifice was pumped to ~1 mTorr by a large diffusion pump. The huge pressure differential between the reaction chamber (at maybe 150 Torr) and the region behind the orifice (1 mTorr) meant the extract gas sample formed a supersonic expansion. The rapid expansion 'froze out' any chemical reactions occuring, preserving the identity of highly reactive species such as radicals and ions, present in small amounts in the bulk plasma. This molecular beam was chopped by a mechanical chopper, to allow phase-sensitive detection and background subtraction.
The final system consisted of a Microwave plasma reactor (right) to which the molecular-beam sampling apparatus was attached. A 100 μm-diameter orifice in the substrate holder (rather than via the viewport) was used to extract a sample of the plasma gas as a supersonic gas expansion. The region immediately behind the orifice was pumped to ~1 mTorr by a large diffusion pump. The huge pressure differential between the reaction chamber (at maybe 150 Torr) and the region behind the orifice (1 mTorr) meant the extract gas sample formed a supersonic expansion. The rapid expansion 'froze out' any chemical reactions occuring, preserving the identity of highly reactive species such as radicals and ions, present in small amounts in the bulk plasma. This molecular beam was chopped by a mechanical chopper, to allow phase-sensitive detection and background subtraction.
The central portion of the expanded gas then passed through a 1 mm-diameter skimmer cone forming a narrow molecular beam. This beam then travelled through a second stage of differential pumping which housed the QMS, supplied by Hiden Analytical. This stage was pumped to ~10-7 Torr by two 70 l/s turbomolecular pumps. The beam then entered the QMS where it was detected.
In theory, the beam detected by the QMS should have the same chemical composition as the bulk plasma. In reality, gas-dynamic effects inherent in the supersonic expansion occurred (such as mass discrimination and background-gas scattering of beam molecules) and had to be corrected for in order to give a correct picture of the CVD process chemistry. The MBMS system was versatile as it could monitor a wide range of species present in the sampling environment, simultaneously, unlike some of the other plasma-chemical diagnostics employed to study diamond CVD (e.g. various types of laser spectroscopy) where only certain species can be detected, and only one at a time. Absolute species mole fractions could be determined when the species of interest were available as a gas and used in the system at a known supplied concentration as a calibrant. The main drawbacks of the molecular beam system were the expense and complexity of building the system.
This system was used to study standard CH4/H2 gas mixtures, as well as the effect upon the chemistry and the diamond film, of additions of chlorine-, nitrogen and phosphorus-containing gases. We also used this system to examine novel gas chemistries, e.g. CO2/CH4 mixtures, and mixtures containing CO/CH4/H2. These are especially important gas mixtures since they allow diamond growth at much lower temperatures than the conventional CH4/H2 mixtures - maybe even as low as 400°C. We also studied Ar/CH4/H2 gas mixtures used to deposit so-called 'ultra-nanocrystalline' diamond films.
Related Papers
- C.A. Rego, P.W. May, C.R. Henderson, M.N.R. Ashfold, K.N. Rosser and N.M. Everitt, "Molecular Beam Mass Spectrometric Study of the Spatial Distribution of Gas-Phase Species Involved in the Growth of HFCVD Diamond Films", in Advances in New Diamond Science and Technology, part of the Proc. 4th Int. Conf. New Diamond Sci. Technol., Kobe, Japan, July 1994, (MYU, Tokyo, 1994), pp.485-488.
- C.A. Rego, P.W. May, C.R. Henderson, M.N.R. Ashfold, K.N. Rosser and N.M. Everitt, "In-situ Mass Spectrometric Study of the Gas-Phase Species Involved in CVD of Diamond as a Function of Filament Temperature", Diamond Relat. Mater. 4, (1995) 770-774.
- C.A. Rego, R.S. Tsang, P.W. May, M.N.R. Ashfold and K.N. Rosser "Gas-Phase Composition Measurements during Chlorine assisted Chemical Vapour Deposition of Diamond: A Molecular Beam Mass Spectrometric Study", J. Appl. Phys., 79 (1996) 7264-73.
- R.S. Tsang, C.A. Rego, P.W. May, J. Thumim, M.N.R. Ashfold, K.N. Rosser, C.M. Younes and M.J. Holt, "Gas-Phase Concentration Measurements and Diamond Film Composition from Chlorine Assisted CVD", Diamond Relat. Mater. 5 (1996) 359.
- R.S. Tsang, C.A. Rego, P.W.May, M.N.R. Ashfold and K.N. Rosser, "Examination of the effects of nitrogen on the CVD diamond growth mechanism using in-situ molecular beam mass spectrometry", Diamond Relat. Mater. 6 (1997) 247.
- R.S. Tsang, P.W. May, M.N.R. Ashfold and K.N. Rosser, "Influence of Phosphine on the Diamond Growth Mechanism: a Molecular Beam Mass Spectrometric Investigation", Diamond Relat. Mater. 7 (1998) 1651.
- S.M. Leeds, P.W. May, E. Bartlett, M.N.R. Ashfold, and K.N. Rosser, "Molecular Beam Mass Spectrometry studies of the gas phase chemistry occurring during Microwave Plasma assisted Chemical Vapour Deposition of Diamond", Diamond Relat. Mater. 8 (1999) 1377-82.
- S.M. Leeds, P.W. May, M.N.R. Ashfold and K.N. Rosser, "Molecular Beam Mass Spectrometry studies of Nitrogen additions to the gas phase during Microwave Plasma Assisted Chemical Vapour Deposition of Diamond", Diamond Relat. Mater., 8 (1999) 226-230
- J.R. Petherbridge, P.W. May, S.R.J. Pearce, K.N. Rosser and M.N.R. Ashfold, "Low temperature diamond growth using CO2/CH4 plasmas: molecular beam mass spectrometry and computer simulation investigations" J. Appl. Phys., 89 (2001) 1484-1492.
- J. Petherbridge, P.W. May, S.R.J. Pearce, K.N. Rosser and M.N.R. Ashfold, "Molecular beam mass spectrometry investigations of low temperature diamond growth using CO2/CH4 plasmas", Diamond Relat. Mater. 10 (2001) 393-398.
- J.R. Petherbridge, P.W. May, G.M. Fuge, G.F. Robertson, K.N. Rosser and M.N.R. Ashfold, "Sulfur doping of diamond films: spectroscopic, electronic and gas-phase studies", J. Appl. Phys. 91 (2002) 3605-3613.
- J.R. Petherbridge, P.W. May, G.M. Fuge, K.N. Rosser and M.N.R. Ashfold, "In situ plasma diagnostics of the chemistry behind sulfur doping of CVD diamond films", Diamond Relat. Mater. 11 (2002) 301-306.
- J.R. Petherbridge, P.W. May, E.J. Crichton, K.N. Rosser and M.N.R. Ashfold, "Sulphur addition to microwave activated CH4/CO2 gas mixtures used for diamond CVD: growth studies and gas phase investigations", Phys. Chem. Chem. Phys. 4 (2002) 5199-5206.
