 Growth of n-doped diamond
Growth of n-doped diamond
|
Doping diamond with boron is relatively straightforward, and allows p-type semiconducting material to be deposited, with the diamond films ranging in conductivity from highly insulating to near metallic. This has enabled the fabrication of many simple p-type devices, such as sensors and electrochemical electrodes, as well as superconducting devices for very heavily boron-doped material. But for more complex devices, such as microprocessors, both p-type and n-type diamond is required. Unfortunately, it has proven extremely difficult to find a dopant for diamond that imparts n-type semiconducting characteristics suitable for use in electronic devices. Nitrogen is one possible dopant and nitrogen-doped diamond has been successfully synthesised using various CVD techniques, including in our lab by adding small amounts of N2 or NH3 to the process gases mixture. However, the nitrogen donor energy level in diamond lies 1.7 eV below the conduction band minimum. This high excitation energy classifies N as a deep donor, and is therefore useless for most electronic devices that operate at room temperature. | 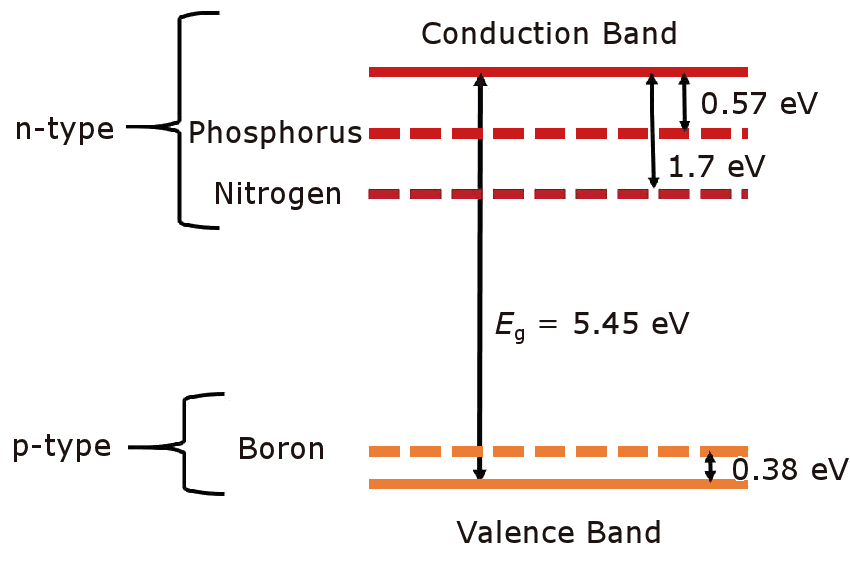 The donor and acceptor levels for B, N and P dopants in diamond. |
Most other potential n-type dopant atoms suffer from being much larger than carbon, so that they cannot easily substitute for C in the rigid diamond lattice. We have previously studied arsenic, antimony and sulfur as possible dopants, but none of these worked due to their very low solid-state solubilities in diamond. Due to its low work-function, lithium has also been suggested as a possible shallow n-type dopant, although it is mobile in diamond above 400 °C so is prone to diffusion (especially down grain boundaries), and can form electrically inactive Li clusters. Moreover, Li only acts as a (poor) n-type dopant if it incorporates interstitially; if Li incorporates substitutionally then it acts as a p-type dopant. Perhaps the most successful n-type dopant to date is phosphorus, but most P-doped films are not sufficiently conducting for many device applications, and there are still only a handful of groups in the world that can produce P-doped diamond reliably.
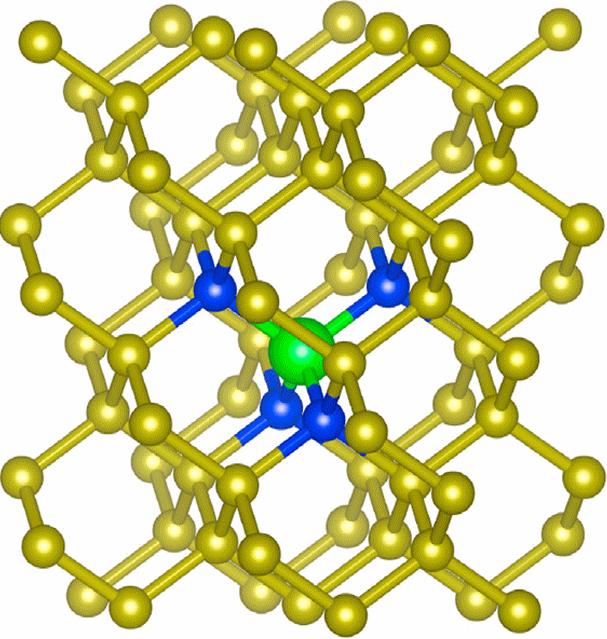 Structure of the LiN4 cluster (with substitutional Li) predicted to show n-type behaviour. C atoms are yellow, N atoms are blue and Li atoms are green. |
Co-dopingOne possible solution is co-doping - incorporation of 2 different dopants simultaneously, either two n-type (e.g. N + Li) or a p-type dopant and an n-type dopant, such as B + N. Our group has done a lot of work in this area, both theoretically and experimentally. DFT calculations predicted that a doping ratio of Li + 4N could form LiN4 clusters which would impart n-type behaviour. However, this has proven very difficult to confirm experimentally due to the problem of Li diffusion and aggregation. In these studies, Li was incoprated by in-diffision into N-doped diamond at high temperatures using Li3N powder as a solid-state dopant. Co-doping using Li + B was also studied, but electrical resistance measurements show that conductivity in all of these co-doped films remains dominated by the B acceptors, while at longer anneal times much of the Li diffused through the diamond and into the Si substrate. Although results so far have been disappointing, there are still many more combinations of dopants to try (P + N, B + P, N + S, etc.), so watch this space... |
Example papers
- P.W. May, M. Davey, K.N. Rosser and P.J. Heard, "Arsenic and antimony doping: An attempt to deposit n-type CVD diamond", Mater. Res. Soc. Symp. Proc. 1039 (2008) 1039-P15-01
- B.S. Truscott, M.W. Kelly, K.J. Potter, M. Johnson, M.N.R. Ashfold, "Microwave Plasma-Activated Chemical Vapour Deposition of Nitrogen-Doped Diamond, I: N2/H2 and NH3/H2 Plasmas", J. Phys. Chem. A 119 (2015) 12962-12976. [doi: 10.1021/acs.jpca.5b09077].
- B.S. Truscott, M.W. Kelly, K.J. Potter, M.N.R. Ashfold, and Yu.A. Mankelevich, "Microwave Plasma-Activated Chemical Vapour Deposition of Nitrogen-Doped Diamond, II: CH4/N2/H2 Plasmas" J. Phys. Chem. A 20 (2016) 8537-8549. pdf [doi: 10.1021/acs.jpca.6b09009].
- J.R. Petherbridge, P.W. May, G.M. Fuge, K.N. Rosser and M.N.R. Ashfold, "In situ plasma diagnostics of the chemistry behind sulfur doping of CVD diamond films", Diam. Relat. Maters. 11 (2002) 301-306.
- J.R. Petherbridge, P.W. May, G.M. Fuge, G.F. Robertson, K.N. Rosser and M.N.R. Ashfold, "Sulfur doping of diamond films: spectroscopic, electronic and gas-phase studies", J. Appl. Phys. 91 (2002) 3605-3613.
- S. Conejeros, M.Z. Othman, A. Croot, J.N. Hart, K.M. O’Donnell, P.W. May, N.L. Allan, "Hunting the elusive shallow n-type donor – an ab initio study of Li and N co-doped diamond", Carbon 171 (2020) 857-868. [doi: 10.1016/j.carbon.2020.09.065].
- M.Z. Othman, P.W. May, N.A. Fox, "In-Situ Incorporation of Lithium and Nitrogen into CVD Diamond Thin Films", Mater. Res. Soc. Symp. Proc. 1511 (2012). [doi: 10.1557/opl.2012.1679]
- M. Z. Othman, P.W. May, N.A. Fox and P.J. Heard, "Incorporation of Lithium and Nitrogen into CVD Diamond Thin Films", Diamond Relat. Mater. 44 (2014) 1-7. [doi: 10.1016/j.diamond.2014.02.001]
- S.C. Halliwell, P.W. May, N.A. Fox, M.Z. Othman, "Investigations of the co-doping of boron and lithium into CVD diamond thin films" Diamond Relat. Mater. 76 (2017) 115-122. [doi: 10.1016/j.diamond.2017.05.001].
- A. Croot, M. Zamir Othman, S. Conejeros, N.A. Fox, N.L. Allan, "A theoretical study of substitutional boron–nitrogen clusters in diamond", J. Phys.: Condens. Matter 30 (2018) 425501. [doi: 10.1088/1361-648X/aade16].
