 Diamond CVD onto different substrates
Diamond CVD onto different substrates
We have spent many years studying the problems associated with growing diamond on a range of different substrate materials. These include standard materials like Si, as well as more novel ones, such as Ti, Ge, W, WC-Co, SiC, SiO2, B4C, AlN, GaN and steels. For the latter we investigated the use of barrier layers to increase adhesion and prevent graphitisation.
Rules for Substrates
In order for CVD diamond to deposit successfully upon a substrate, the material needs to have the following properties:
- The substrate must have a melting point (at the process pressure) higher than the temperature required for diamond growth, normally > 700 °C. Unfortunately, this precludes the use of existing CVD techniques to coat low-melting-point materials, like plastics, aluminium, some glasses, and electronic materials such as GaAs.
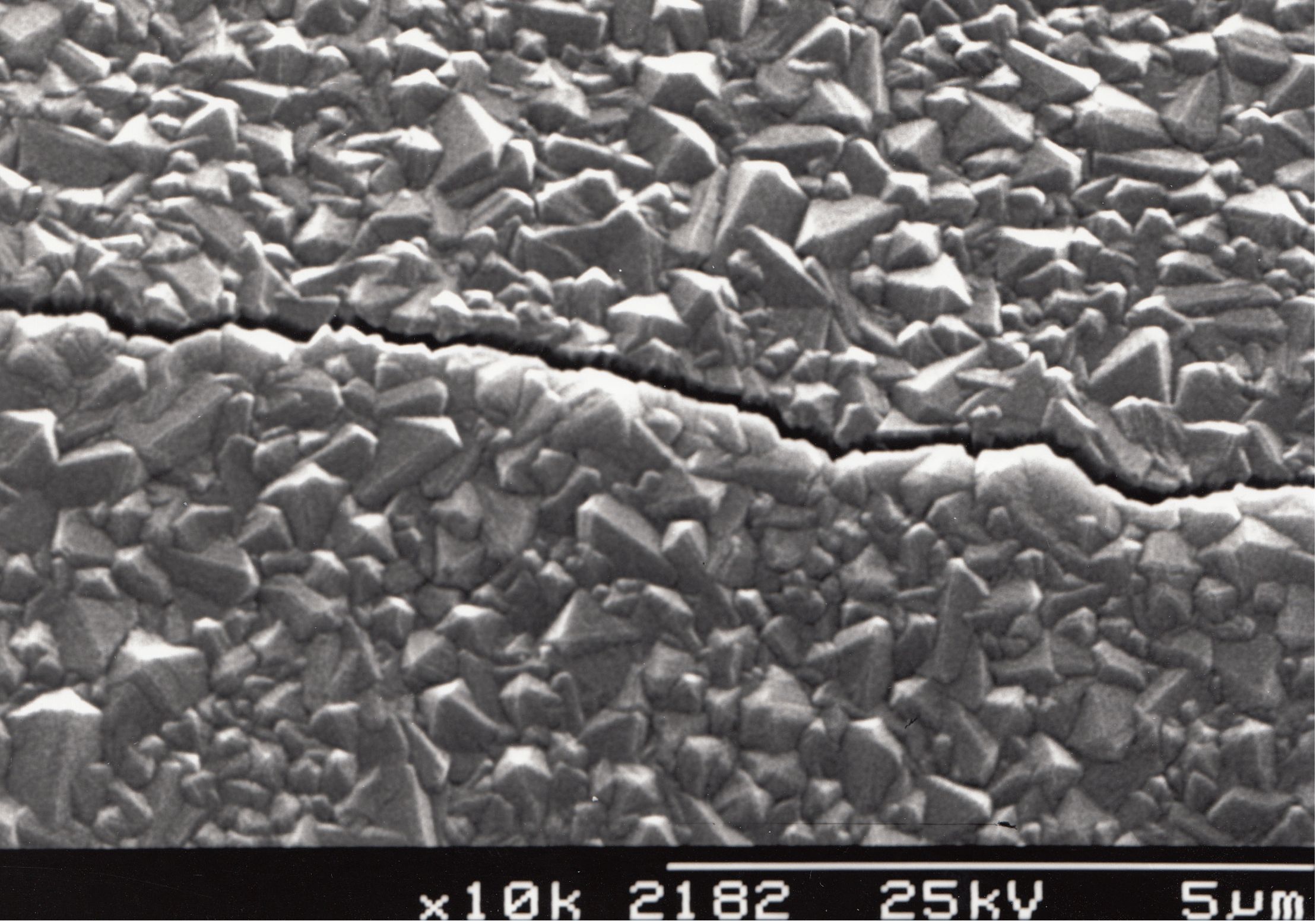
- The substrate material should have a thermal expansion coefficient comparable with that of diamond. This is because at the high growth temperatures currently used, a substrate will tend to expand, and thus the diamond coating will be grown upon and bonded directly to an expanded substrate. Upon cooling, the substrate will contract back to its room temperature size, whereas the diamond coating, with its very small expansion coefficient, will be relatively unaffected by the temperature change. Thus, the diamond film will experience significant compressive stresses from the shrinking substrate, leading to bowing of the sample, and/or cracking, flaking or even delamination of the entire film (as seen in the picture below).
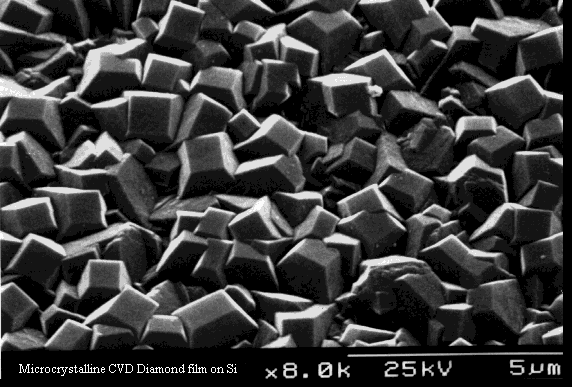
A continuous microcrystalline diamond film grown on Si.
A microcrystalline diamond film grown on quartz that has
cracked due to thermal mismatch between the diamond and substrate. - The substrate material be capable of forming a carbide layer to a certain extent. This is because diamond CVD on non-diamond substrates usually involves the formation of a thin carbide interfacial layer, upon which the diamond then grows. The carbide layer can be pictured as the ‘glue’ which promotes growth of diamond and aids its adhesion by (partial) relief of stresses at the interface (caused by lattice mismatch or substrate contraction). There are 3 cases:
-
- Little or no solubility or reaction with C. These materials do not form a carbide layer, and so any diamond layer will not adhere well to the surface. This can be used as one method to make free-standing diamond films, as the films will often readily delaminate after deposition. This category includes metals such as Cu, Sn, Pb, Ag and Au, as well as non-metals such as Ge, sapphire, alumina.
- Substantial mutual solubility or reaction with C. In these materials, the substrate acts as a carbon sink, and deposited carbon dissolves into the surface, forming a solid solution. This can result in large quantities of C being transported into the bulk, rather than remaining at the surface where it can promote diamond nucleation. Often diamond growth only begins after the substrate is saturated with carbon, and this can dramatically affect the physical properties of the resulting composite. Metals where this is significant include Pt, Pd, Rh, Ni, Ti and Fe. The last metal is of particular concern, because this means that at present all industrially important ferrous materials (such as iron and stainless steel) cannot be diamond coated using simple CVD methods.
- Limited carbide formation. These include metals such as Mo, W and certain other rare-earth metals, and adherent diamond films generally grow well on these. Non-metals, such as B or Si, and Si-containing compounds such as SiO2, quartz and Si3N4, also form carbide layers. Substrates composed of carbides themselves, such as SiC, WC and TiC are particularly amenable to diamond deposition.
- Little or no solubility or reaction with C. These materials do not form a carbide layer, and so any diamond layer will not adhere well to the surface. This can be used as one method to make free-standing diamond films, as the films will often readily delaminate after deposition. This category includes metals such as Cu, Sn, Pb, Ag and Au, as well as non-metals such as Ge, sapphire, alumina.
Example papers
- C.A. Rego, P.W. May, E.C. Williamson, M.N.R. Ashfold, Q.S. Chia, K.N. Rosser and N.M. Everitt, "CVD Diamond Growth on Germanium for Infra-Red Window Applications", Diamond Relat. Mater., 3 (1994) 939-941.
- P.W. May, C.A. Rego, C.G. Trevor, E.C. Williamson, M.N.R. Ashfold, K.N. Rosser and N.M. Everitt, "Deposition of Diamond Films on Sapphire: Studies of Interfacial Properties and Patterning Techniques", Diamond Relat. Mater. 3 (1994) 1375.
- P.W. May, K.N. Rosser, N.A. Fox, C.M. Younes and G. Beardmore, "Deposition of CVD Diamond onto Boron Carbide Substrates", Diamond Relat. Mater. 6 (1997) 450.
- P.W. May, H.Y. Tsai, W.N. Wang and J.A. Smith, "Deposition of CVD Diamond onto GaN", Diam. Relat. Maters. 15 (2006) 526-530.
- F. Brannan, P.W. May, S.C. Halliwell and L. Payne, "Deposition of CVD diamond onto Zirconium", Mater. Res. Soc. Symp. Proc. 134 (2015) [doi: 10.1557/opl.2015.174].
