|
Home
The molecule Physical properties Chemical properties Antimicrobial properties Structure-activity relationships Biosynthesis Chemical syntheses References |
The molecule
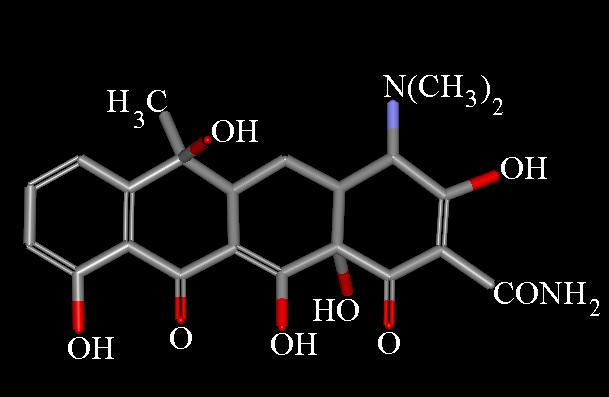
Presently, the name "tetracycline" refers to a number of antibiotics of either natural, or semi-synthetic origin, derived from a system of four linearly annelated six-membered rings (1,4,4a,5,5a,6,11,12a-octahydronaphthacene) with a characteristic arrangement of double bonds. The tetracycline molecule possesses five asymmetric centers: C-4, -4a, -5a, -6, and -12a. A determination of the crystal structure of aureomycin hydrochloride has clearly defined the relative stereochemistry of these centers [22]. 1H NMR studies [23,24], especially as applied to 5-hydroxytetracycline, have established independently the proton topology [8]. The determination of its complete structure, involving a sagacious use of IR and UV spectroscopy, has been named a masterpiece in the field of structural analysis [28].