|
Home
The molecule Physical properties Chemical properties Antimicrobial properties Structure-activity relationships Biosynthesis Chemical syntheses References |
Chemical syntheses
The tetracycline molecule has offered a splendid challenge to a synthetic organic chemist,
a mojor obstacle in its total synthesis being the stereospecific
introduction of many functional groups into the basic carbon nucleus. Of even greater
concern is the extreme chemical sensitivity of this molecule, particularly its lability
in acidic and basic media [29].
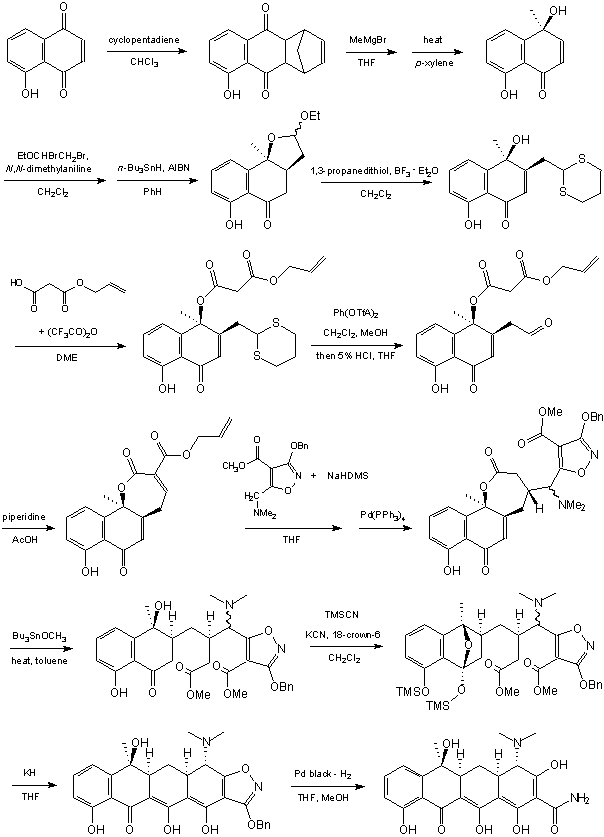
The first synthesis of a tetracycline-like molecule with the functionality necessary for
antimicrobial properties was accomplished by the legendary
Robert B. Woodward and a group at Pfizer [12]
(Some earlier work utilized completely aromatic precursors in conventional acylation
reaction to form the C(11), C(12) b-diketone system [14,15]).
The product, sancycline (6-demethyl-6-deoxytetracycline), is an active antibiotic, but not
used as often as aureomycin or terramycin. The Woodward's synthesis is completely linear
and involves incorporation of the 4-dimethylamino- group and the 12a-hydroxy- group into
the final structure. The synthesis provides a dramatic illustration of the importance and
creativity of organic chemistry [13].
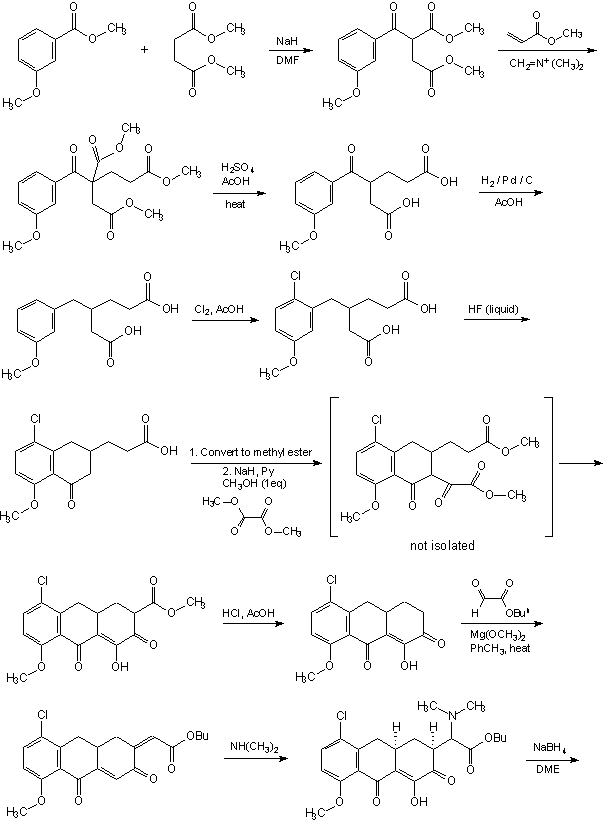
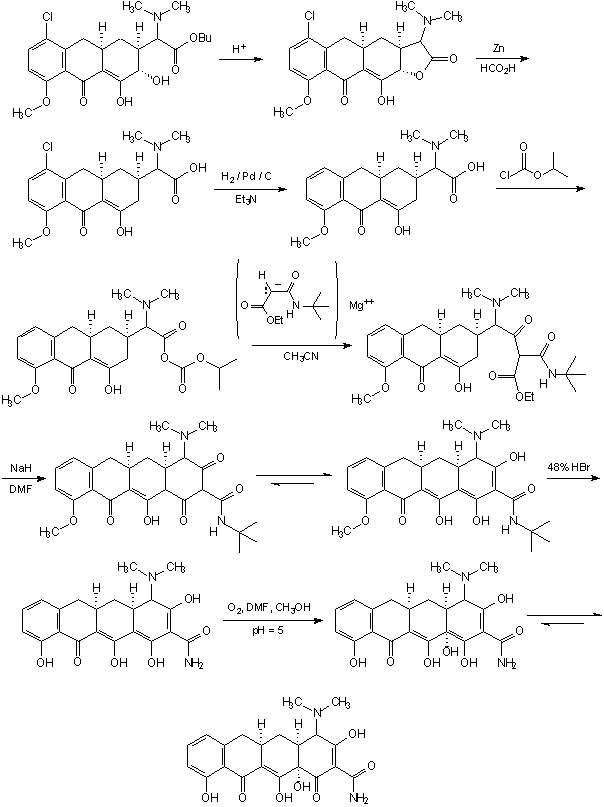
The following synthesis was reported by Shemyatkin et al. [16]. The precursor had been prepared in six stages from juglone (a naturally occurring quinone, the active staining principle of black walnut hulls) [17], and the product was obtained by degradation of the naturally occurring tetracycline according to the procedure of Green and Boothe [18]. Since the latter is a degradation product of tetracycline, and has been reconverted by 12a-hydroxylation followed by photo-addition etc., according to the Scott procedure [19], into tetracycline, a formal total synthesis of the latter had been accomplished [8].
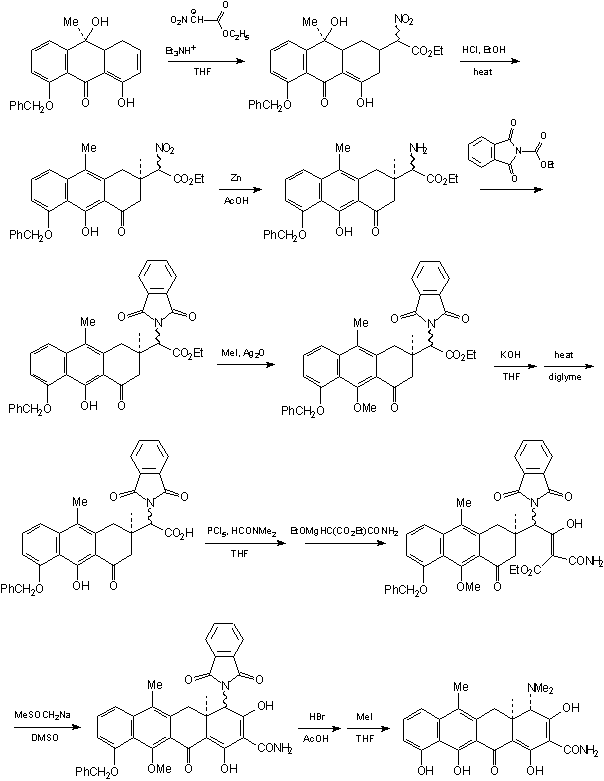
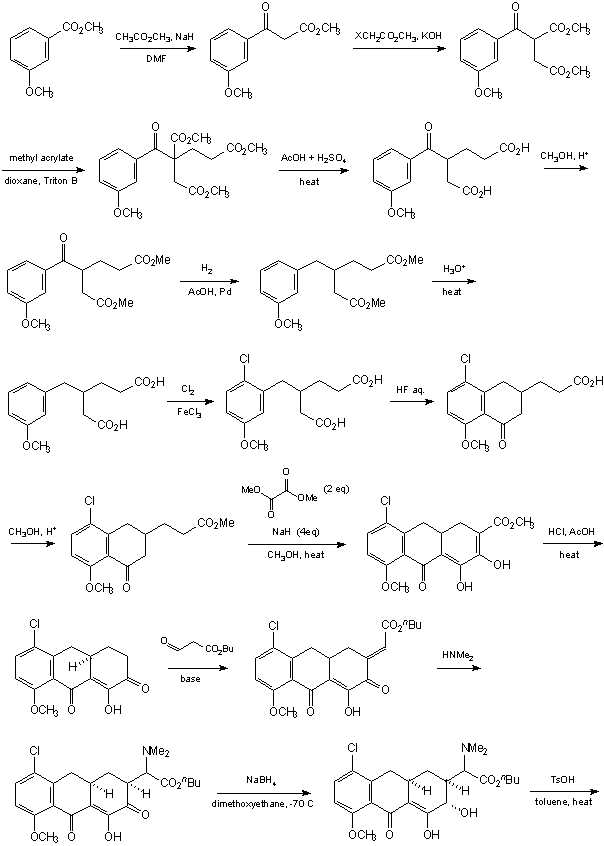
The next synthesis of a tetracycline-like structure by Woodward was reported in 1968 [5]:
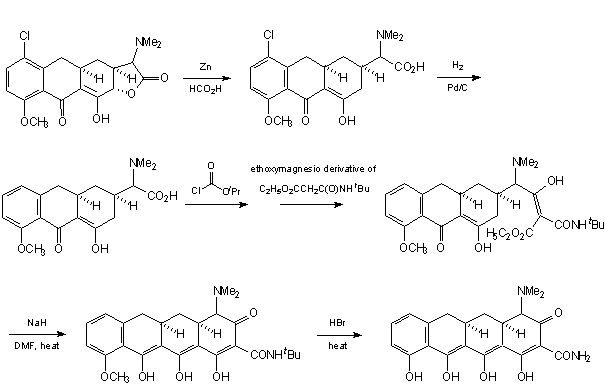
Muxfeldt et al. presented a simple route to tetracycline system, using brilliant condensation and oxazolone moiety cleavage to produce a sensitive group in A ring [29]:
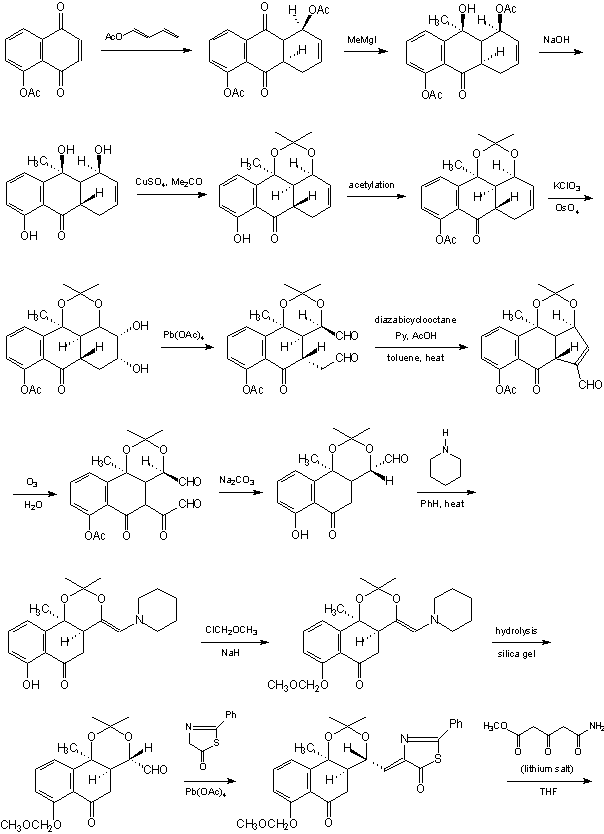
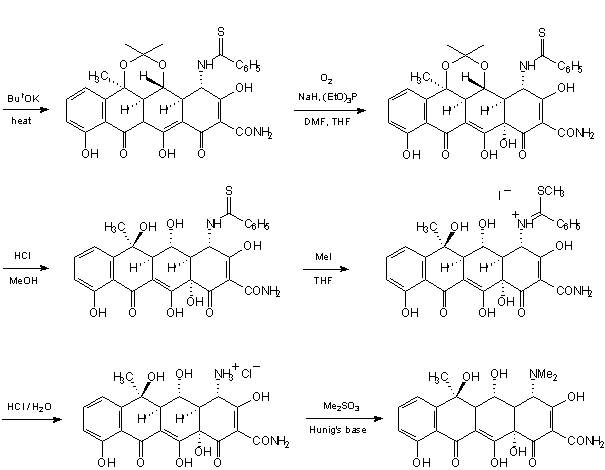
The improved method of the latter, with a controlled introduction of stereocenter at C4a, has been presented by Stork et al. [30]: